June 21, 2011 – A study introduced at the Society of Nuclear Medicine’s (SNM) 58th Annual Meeting may lead to the next wave of cancer imaging by helping to develop a molecular imaging agent that detects many malignant cancers’ incessant development of blood vessels — a process called angiogenesis. A protein biomarker known as CD105 has been shown to indicate tumor angiogenesis in cancer patients.
“Noninvasive molecular imaging is a critical component of 21st century personalized medicine, and one of the hallmarks of cancer is angiogenesis,” says Weibo Cai, Ph.D., assistant professor of radiology, medical physics and biomedical engineering at the University of Wisconsin–Madison’s School of Medicine and Public Health. “CD105 is considered by many to be the best biomarker for evaluating tumor angiogenesis. Noninvasive imaging of this protein’s expression could potentially play a variety of roles in the future of cancer patient management. CD105-targeted imaging agents also represent a new paradigm for the assessment of cancer therapies that target tumor angiogenesis. Applications for this agent could reach far beyond cancer and open many new avenues for future research.”
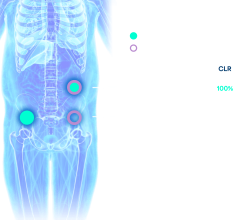
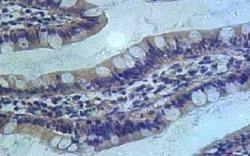
Malignant cancers are defined by their ability to grow like weeds, forming fast and strong networks of blood vessels that carry oxygen and nutrients to the cancer’s insatiable cellular structure. Endoglin, or CD105, is a naturally occurring protein that resides on the cell’s surface. Above-normal expression of this protein is associated with poor cancer prognosis in more than 10 solid tumor types. The clinical standard for evaluating tumor angiogenesis is microvessel density (MVD) analysis, which is conducted by staining CD105 in tumor tissues that have been obtained by either surgical removal or biopsy. This study represents the first of its kind to report preliminary data on the noninvasive imaging of CD105 expression with positron emission tomography (PET), which provides a reliable measure of angiogenesis in the tumor.
Researchers used the medical isotope Copper-64 (Cu64) to label an antibody called TRC105, which binds to CD105. The full name of the agent is (64)Cu-DOTA-TRC105. The TRC105 antibody is currently being studied in a U.S. multicenter phase 1 human trial, and multiple phase 2 therapy trials are planned or already underway for a range of cancer types. The current study specifically marks the effectiveness of using 64Cu-DOTA-TRC105 to gauge tumor angiogenesis. Results of the study showed this PET imaging agent to be highly effective, with rapid and persistent CD105-targeted uptake by tumors in mice.
Not only could this potentially be a turning point for cancer imaging and therapy, but some other major causes of death like heart attack, stroke and atherosclerosis also actively demonstrate the over-expression of CD105. Molecular imaging of this protein could one day lead to expanded tools for the detection and treatment of any number of diseases characterized by enhanced angiogenesis.
Scientific Paper 296: Y. Zhang, H. Hong, Y. Yang, J. Engle, T. Barnhart, R. Nickles, B. Leigh, W. Cai, University of Wisconsin Madison, Madison, Wis.; Tracon Pharmaceuticals Inc., San Diego, Calif.; “Positron emission tomography imaging of CD105 expression during tumor angiogenesis.” SNM’s 58th Annual Meeting, June 4–8, 2010, San Antonio, Texas
For more information: www.snm.org


 January 27, 2026
January 27, 2026