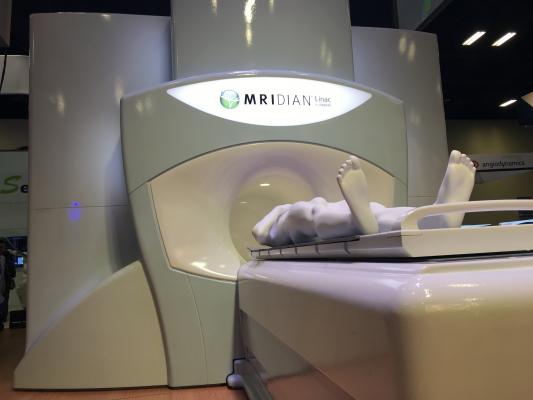
August 19, 2019 — ViewRay Inc. announced the acceptance of publication by the International Journal of Radiation Oncology, Biology and Physics of the first prospective clinical trial of MR-guided radiation therapy (MRgRT) in patients with localized prostate cancer. This robust study of clinician and patient reported outcomes demonstrated zero CTCAE v4 grade 3 or higher gastrointestinal (GI) and genitourinary (GU) toxicity and even lower incidence of grade 2 toxicity than investigators hypothesized. It is also one of the first prospective clinical trials to study stereotactic body radiation therapy (SBRT) in a mix of intermediate- and high-risk prostate cancer patients, a challenging patient population to treat. The journal is the official scientific journal of the American Society for Radiation Oncology (ASTRO).
Researchers from Amsterdam University Medical Center enrolled 101 patients with intermediate- or high-risk prostate cancer in a prospective phase II clinical trial. All patients received MRgRT in five fractions of 7.25 Gy to the target volume using on-table adaptive techniques. The trial did not use implanted markers or tissue spacers because treatments were delivered under magnetic resonance (MR)-guidance, thereby eliminating an invasive procedure, potentially associated complications and implantation costs.
Results at three months showed that no early CTCAE v4.0 grade 3 GU or GI toxicity was observed, and the maximum cumulative grade 2 early GU and GI toxicity measured by any symptom at any study time point was 23.8 percent (study hypothesis 40 percent) and 5 percent (study hypothesis 15 percent). These results were obtained in a complex clinical cohort (59.4 percent high-risk patients) and are comparable to what would be typically observed in lower-risk populations, pointing to the potential benefits of MR-guided SBRT in this higher risk group. Additionally, the low incidence of early GI toxicity, despite the inclusion of the base of the seminal vesicles in 96 percent of patients, illustrates the benefit of MR-guidance and on-table adaptive re-planning. This technology facilitates smaller treatment margins while minimizing damage to surrounding tissue and critical structures, such as urethra, rectum and bladder. The publication noted that incontinence was uncommon, reported by 4 percent of patients at the end of MRgRT and decreasing over time.
"SBRT offers significant promise in the treatment of prostate cancer. Our clinical trial takes that a step further in showcasing its value in patients with intermediate- and high-risk disease, with a focus on evaluating associated toxicities and quality of life outcomes," said principal investigator Anna Bruynzeel, M.D., Ph.D., radiation oncologist at Amsterdam UMC. "We see a lower incidence of GI and GU toxicity with MR-guidance as compared to similar SBRT prostate cancer studies. The results reinforce the value of MRIdian's real-time on-table adaptive treatment with automatic beam gating for prostate patients."
For more information: www.redjournal.org
Related Content
VIDEO: Walk Through of the MRI Radiotherapy System at Henry Ford Hospital
FDA Clears Advancements for Viewray MRIdian Radiation Therapy System
Reference
Bruynzeel A.M.E., Tetar S.U., Oei S.S., et al. A prospective single-arm phase II study of stereotactic magnetic-resonance-guided adaptive radiotherapy for prostate cancer: Early toxicity results. International Journal of Radiation Oncology Biology Physics, published online Aug. 13, 2019. https://doi.org/10.1016/j.ijrobp.2019.08.007


 March 09, 2026
March 09, 2026