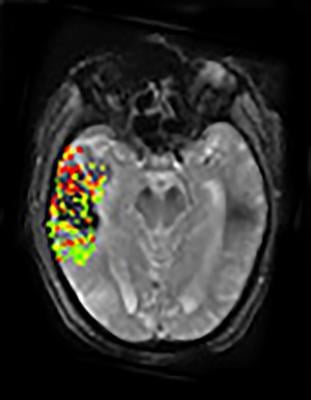
June 29, 2016 — In a study of stroke patients, investigators confirmed through magnetic resonance imaging (MRI) brain scans an association between the extent of disruption to the brain’s protective blood-brain barrier and the severity of bleeding following invasive stroke therapy. The results of the National Institutes of Health-funded study were published in Neurology.
These findings are part of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE)-2 Study. The study was designed to see how MRIs can help determine which patients undergo endovascular therapy following ischemic stroke caused by a clot blocking blood flow to the brain. Endovascular treatment targets the ischemic clot itself, either removing it or breaking it up with a stent.
The blood-brain barrier is a layer of cells that protects the brain from harmful molecules passing through the bloodstream. After stroke, the barrier is disrupted, becoming permeable and losing control over what gets into the brain.
“The biggest impact of this research is that information from MRI scans routinely collected at a number of research hospitals and stroke centers can inform treating physicians on the risk of bleeding,” said Richard Leigh, M.D., a scientist at NIH’s National Institute of Neurological Disorders and Stroke (NINDS) and an author on the study.
In this study, brain scans were collected from more than 100 patients before they underwent endovascular therapy, within 12 hours of stroke onset. Leigh and his team obtained the images from DEFUSE-2 investigators.
Using a new method of image processing, Leigh’s group was able to get detailed measurements on the extent to which the blood-brain barrier is disrupted following a stroke. Combining that data with findings from the DEFUSE-2 study revealed that large degrees of blood-brain barrier disruption were associated with severe bleeding following endovascular therapy. Extensive breakdown of the blood-brain barrier was associated with parenchymal hematoma, a form of bleeding in the brain that carries the greatest risk for the patient. In addition, the results showed a link between the location of blood-brain barrier damage and post-treatment brain bleeding.
Ischemic stroke patients are increasingly receiving combination therapy, endovascular treatment along with an intravenous drug known as tissue plasminogen activator (t-PA), to effectively break up clots in the brain. However, bleeding into the damaged brain tissue is a serious complication of both acute stroke therapies. t-PA has been shown to be most effective when given within a few hours of stroke onset, but the treatment window for endovascular therapy is unknown.
“With the growing precision of brain imaging technology, researchers are able to get a detailed look at what is going on in the brain during a stroke. Innovative studies, such as DEFUSE-2, can help patients and their doctors make more informed decisions about medical care,” said Walter Koroshetz, M.D., director of NINDS.
According to the authors, examining blood-brain barrier disruption on brain images may potentially help doctors identify patients not likely to benefit from endovascular therapy. “It is too early to say how these images will be able to help guide clinical decisions, but they can expand how we think about stroke, especially as we try to broaden treatment options for this disease that can have devastating consequences,” said Leigh.
The DEFUSE-3 trial is currently underway, in which researchers will use imaging data to select patients for endovascular therapy up to 16 hours after stroke onset. The patients’ recovery will be closely monitored for three months following the treatment.
This study was supported by the NIH (NS039325, NS051372, NIH Intramural Program).
For more information: www.nih.gov


 February 13, 2026
February 13, 2026 









