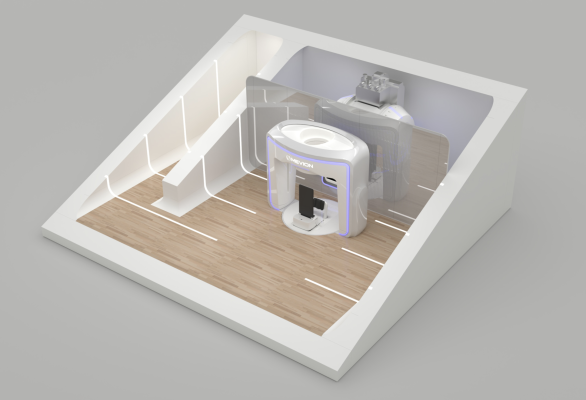
The MEVION S250-FIT Proton Therapy System (Photo: Business Wire)
November 3, 2022 — Mevion Medical Systems, the leading provider of compact proton therapy systems for use in radiation treatment for cancer patients, announced today that it is developing the MEVION S250-FIT Proton Therapy System with HYPERSCAN Pencil Beam Scanning (PBS). Stanford Health Care has been selected as the first site where the system will be developed and installed.
Proton therapy is a precise form of radiation therapy that reduces the amount of unnecessary radiation exposure to healthy tissue, which has the potential to decrease or eliminate the treatment side effects and lessen the risk of secondary malignancies.
Historically, the size, cost, and inherent complexity of proton technologies have limited the adoption of proton therapy. Mevion believes that the MEVION S250-FIT system has the potential to overcome the practical challenges of other technologies because the system is designed to be a more compact, affordable solution that can fit into an existing LINAC vault. Today, new proton therapy centers in the U.S. are almost exclusively compact single-room systems. Mevion’s compact system would continue to advance the design and accessibility of proton therapy. The MEVION S250-FIT system is currently in development and has not received regulatory clearance for clinical use.
“Mevion is dedicated to making proton therapy deployment similar to conventional radiation by simplifying room renovation requirements, enabling faster installation, and facilitating integration with other radiation therapy modalities,” said Tina Yu, Ph.D., CEO and President of Mevion Medical Systems. “Through our arrangement with Stanford Health Care, Mevion is aiming to demonstrate that proton therapy can fit in an existing vault of a radiation oncology department and in turn become more accessible.”
For more information: www.mevion.com
*The MEVION S250-FIT Proton Therapy System has not received regulatory clearance.


 February 04, 2026
February 04, 2026 









