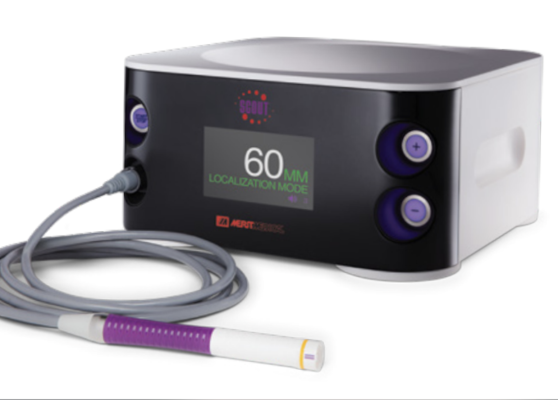
February 15, 2024 — Merit Medical Systems, Inc., a global leader of healthcare technology, has received US Food and Drug Administration (FDA) 501(k) clearance for the SCOUT MD Surgical Guidance System.
The release of the new guidance system demonstrates Merit’s ongoing leadership in oncology and marks a significant advancement in breast cancer care. SCOUT MD broadens Merit’s oncology portfolio, designed to enhance diagnosis and treatment of breast and other soft tissue cancers. Products include the SCOUT Radar Localization System with SCOUT Mini Reflector and SCOUT Bx Delivery System as well as the SAVI Brachy System.
A breakthrough solution, SCOUT MD supports implantation of up to four different reflector configurations. When implanted within abnormal breast or other soft tissue, the reflectors are designed to pinpoint tumor location in multiple dimensions for more precise excision. A targeted approach can help minimize damage to surrounding healthy tissue, decrease the likelihood of re-excision, and avoid the emotional and physical trauma associated with a second surgery.
“Merit’s development of different reflectors with different signals will allow surgeons to better delineate the edges of resection,” said John Vincent Kiluk, MD, FACS, Breast Surgical Oncologist at the Moffitt Cancer Center and Professor of Oncologic Sciences at USF, Tampa, Florida. “The subsequent result of this surgical precision should improve a surgeon’s ability to obtain adequate margins on larger tumors and decrease the need for repeat surgery.”
Every fourteen seconds, a woman is diagnosed with breast cancer.1 In 2020, 2.3 million women were diagnosed, and 685,000 died of breast cancer globally, making it the world’s most prevalent cancer.2 Lumpectomy (a type of breast-conserving surgery) is often performed as treatment. However, an estimated 20% ̶ 30% of women who undergo lumpectomy will need a repeat surgery.3 Localization procedures help surgeons precisely target breast cancer, which may result in more successful surgeries and improved patient outcomes.
The release of SCOUT MD coincides with an important milestone for Merit and breast surgery patients worldwide. As of January 2024, Merit has distributed more than 500,000 devices for placement in patient procedures.
“This momentum has enabled us to collect valuable real-world evidence through physician feedback, giving us a better understanding of what’s needed to improve oncology care,” said Fred P. Lampropoulos, Merit’s Chairman and CEO. “In turn, we’re developing new technologies, like SCOUT MD, that enhance the patient experience and reduce the burden breast cancer places on women worldwide. We’re honored to be a part of their care journey, and we intend to continue innovating for them.”
For more information: https://www.merit.com/
References
1. Breast Cancer Research Foundation. 2023. “Breast Cancer Statistics and Resources.” https://www.bcrf.org/breast-cancer-statistics-and-resources/
2. World Health Organization. 26 Mar 2021. “Breast Cancer.” https://www.who.int/news-room/fact-sheets/detail/breast-cancer
3. Havel et al. 2019. “Impact of the SSO-ASTRO Margin Guideline on Rates of Re-Excision after Lumpectomy for Breast Cancer: a Meta-Analysis.” Annals of Surgical Oncology 26 (5): 1238 ̶ 1244. (PMID: 30790112)


 February 18, 2026
February 18, 2026 









