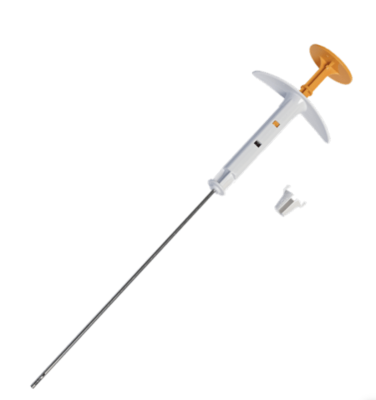
February 23, 2023 — Merit Medical Systems, Inc., a leading global manufacturer and marketer of healthcare technology, announced today the FDA has granted Breakthrough Device Designation for the SCOUT MD Surgical Guidance System.
The SCOUT MD is the latest step in Merit’s ongoing commitment and leadership in advancing oncology care. SCOUT MD expands Merit’s portfolio of products to optimize oncology for breast and other soft tissue cancers, which includes the SCOUT Radar Localization system with new SCOUT Mini Reflector and SCOUT Bx Delivery System, and the SAVI Brachy System.
A first of its kind, the SCOUT MD localization system supports implantation of up to four unique reflector configurations. When implanted within abnormal breast or other soft tissue, the reflectors enable surgeons to pinpoint tumor location in multiple dimensions for more precise excision, minimizing trauma to healthy tissue and helping to reduce the likelihood of re-excision and the emotional and physical pain associated with a second surgery.
Breast cancer is the most common cancer worldwide, representing 12.5% of all new annual cancer cases.1 Lumpectomy (a type of breast conserving surgery) is often performed as treatment. However, an estimated 20% to 30% of women who undergo lumpectomy will need a repeat surgery.2 Localization procedures help surgeons precisely target breast cancer, which may result in more successful surgeries and improved patient outcomes.
“This designation recognizes the significant contribution we believe SCOUT MD can make in oncology treatment,” said Fred P. Lampropoulos, Merit Medical’s Chairman and CEO. “A diagnosis of cancer is devastating to patients and their loved ones. Merit is committed to delivering new technologies to improve outcomes and enhance the patient experience in cancer care. SCOUT MD is designed to help increase the precision with which surgeons can operate, supporting tissue-conserving treatment and reduced reintervention. We look forward to working with the FDA to make this important technology available to patients.”
FDA Breakthrough Device Designation is intended to help patients receive timely access to technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases or conditions. Under the program, the FDA provides priority review and interactive communication regarding device development and clinical trial protocols, through to commercialization decisions.
For more information: https://www.merit.com/
References
- BreastCancer.org. 2023. “Breast Cancer Facts and Statistics.” Last modified January 18, 2023. https://www.breastcancer.org/facts-statistics
- Havel, Liska et al. 2019. “Impact of the SSO-ASTRO Margin Guideline on Rates of Re-excision After Lumpectomy for Breast Cancer: a Meta-analysis.” Annals of Surgical Oncology 26 (5): 1238 ̶ 1244.


 February 18, 2026
February 18, 2026 









