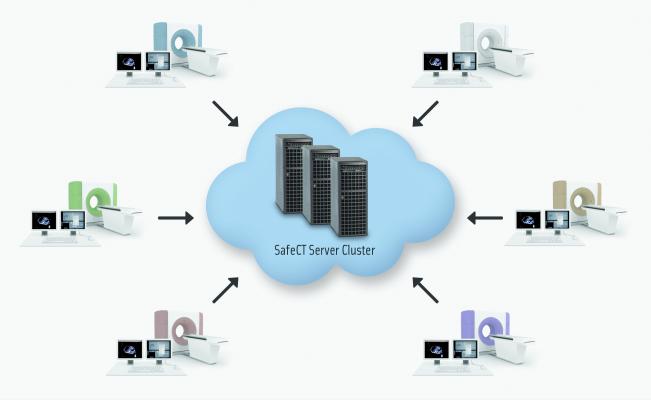
November 13, 2012 – Tirat Carmel, Israel – Medic Vision Imaging Solutions Ltd., today announced the release of SafeCT Enterprise Edition, a fail-safe, scalable, high-performance version of SafeCT designed for organizations with a large number of CT scanners. SafeCT Enterprise Edition comprises a cluster of SafeCT servers working together to deliver top-notch diagnostic quality to CT studies acquired with low-dose protocols on any number of existing scanners in multiple locations on a network.
Utilizing a cluster of SafeCT servers and proprietary Grid Computing technology, the Enterprise Edition provides continuous real-time iterative image reconstruction functionality to all CT scanners on a network through fail-over, load balancing, parallel processing, and centralized management. This guarantees that there is no single point of failure and no delays in image processing for any of the scanners. The Enterprise Edition is scalable, accommodating incremental growth, and is highly cost-effective. SafeCT Enterprise Edition is a first for the industry and will be featured at the RSNA Annual Meeting in Chicago, November 25-30, 2012 at McCormick Place, Hall A, Booth 4444.
Using proprietary patented iterative volumetric image reconstruction algorithms, SafeCT has delivered high-quality images for more than 50,000 CT studies covering a wide range of exposure parameters, eliminating the need to upgrade existing CT systems individually. The technology is helping to cut costs in major hospitals nationwide on all CT scanner systems from major manufacturers including GE, Philips, Siemens and Toshiba.
According to ECRI Institute’s August 2012 Health Devices article, “Optimizing CT Dose”: "SafeCT may offer a more affordable option for facilities looking to provide iterative image reconstruction for a large number of CT scanners, compared to upgrading individual scanners. We highly recommend considering this system for new and existing CT systems that do not already have iterative reconstruction."
For more information: www.atlantisworldwide.com


 February 09, 2026
February 09, 2026 









