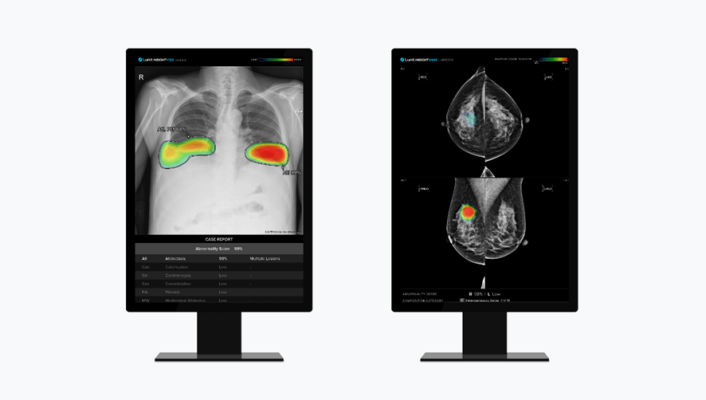
(from left to right) Lunit's AI-powered chest X-ray analysis solution 'Lunit INSIGHT CXR' and AI-powered mammography analysis solution 'Lunit INSIGHT MMG'
November 6, 2023 — Lunit, a leading provider of AI-powered solutions for cancer diagnostics and therapeutics, is set to unveil seven significant abstracts at the Radiological Society of North America (RSNA) 2023 Annual Meeting, taking place from November 26 to November 30 in Chicago.
The highlighted abstracts for RSNA 2023 include:
- an oral presentation: Lunit's AI model redefines the efficiency of chest radiograph reporting by combining new and existing AI algorithms to filter normal chest radiographs and act as a safety net to reduce radiologists’ workload.
- an oral presentation: in collaboration with a Swedish research group, the accuracy and robustness metrics of Lunit INSIGHT MMG were assessed in comparison to radiologists.
- an ePoster: Lunit's novel, predictive AI model goes beyond traditional mammographic density by investigating mammographic parenchymal patterns and longitudinal changes as predictive markers for breast cancer risk.
- an ePoster: comparison of the diagnostic accuracy of radiologists with and without Lunit’s AI-powered breast cancer screening solution.
- an ePoster: assessment of the value of supplemental breast ultrasound and Lunit’s AI in screening mammography for women with dense breasts.
- an ePoster: assessment of the false-negative results of Lunit’s AI-based CADe/x in mammograms for invasive breast cancers, considering molecular subtypes.
"Lunit is consistently backing the effectiveness of the Lunit INSIGHT suite by participating in RSNA and unveiling a spectrum of innovative research results each year at RSNA since 2016. We remain dedicated to enhancing our solutions, steering the field towards a new era of chest and breast cancer screening," said Brandon Suh, CEO of Lunit.
Beyond abstract presentations, Lunit will further showcase its AI solutions onsite by setting up a dedicated booth (#4165) at the AI Showcase section. Visitors will have the opportunity to experience firsthand how Lunit’s AI solutions are contributing to more than 2000 medical institutions worldwide and addressing the most significant global health challenges.
For more information: www.lunit.com
Find more RSNA23 conference coverage here


 February 18, 2026
February 18, 2026 









