July 27, 2016 — A U.K-Poland consortium was recently awarded a €1.1M grant to develop a magnetic resonance imaging (MRI)-based medical device to diagnose liver disease in children. Such a device could save children in the future from having to undergo biopsies.
One in five children in Europe suffer from fatty liver disease, but clinical symptoms only surface at later stages when treatment options are limited. Early detection would have a massive impact on how this disease is managed, but the current diagnostic is a painful, invasive biopsy. "Liver biopsy is important for making diagnosis of various liver diseases and is frequently used in follow up to make decisions on therapy. We try to decrease indications for liver biopsy in children because it can cause complications and needs sedation and anesthesia," explained Prof. Piotr Socha, from the Children's Memorial Health Institute in Warsaw, Poland.
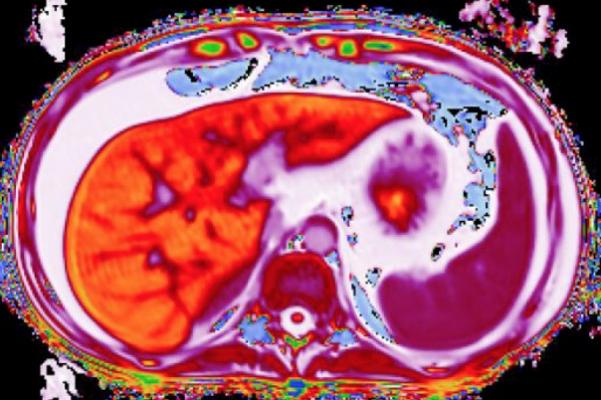
LiverMultiScan uses a painless, 15-minute MRI scan which characterizes liver tissue. Developed by Perspectum Diagnostics in Oxford, U.K., this test already has regulatory clearance for use in adults to aid physicians in diagnosing early liver disease.
Supported by funding from EUREKA/Eurostars, the Kids4LIFe consortium will develop and validate LiverMultiScan specifically for patients under 16. In a clinical trial, children who would normally undergo liver biopsy as part of their usual care will also be offered a LiverMultiScan. The information gathered from this study will help the consortium understand how and when the technology is best used in the diagnosis of pediatric liver disease.
As part of the project, a cloud-based health data management system will be developed by the Polish telemedicine company Silvermedia. This platform will allow rapid transfer of liver scans, as well as other health information and diagnostic test results, between local pediatricians and experts in other cities and countries.
LiverMultiscan is now installed in medical institutions on three continents. Used in the clinical management of patients with chronic liver disease, the technology offers a quantitative liver assessment in a safe, non-invasive 15-minute MRI scan. Analysis is based on assessment of native properties of liver tissue, accurate measurement of liver fat and other metrics. The technology is currently being used to assess primary endpoints in clinical trials for investigational therapies to treat non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
LiverMultiScan is manufactured by Mirada Medical, and has CE-marking and U.S. Food and Drug Administration (FDA) clearance.
For more information: www.perspectum-diagnostics.com


 February 13, 2026
February 13, 2026 









