November 23, 2022 — KA Imaging’s innovative Reveal 35C flat panel detector has received EU MDR CE Mark Certificate from the EU Notified Body. The X-ray detector, powered by patented SpectralDR technology, is now qualified for sale in any of the 27 countries of the European Union, plus the four countries that are part of the European Free Trade Association (EFTA).
“It’s a very exciting moment for us at KA Imaging,” said Amol Karnick, President and CEO of KA Imaging. “We are engaged in conversations with many clinical sites in Europe, and the CE Mark will allow us to make installations and start patient imaging,” said Karnick.
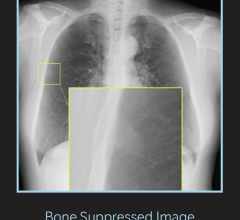
KA Imaging’s SpectralDR technology enables dual-energy subtraction, providing bone and tissue differentiation with a single standard X-ray exposure. It acquires three images simultaneously (DR, bone and soft tissue dual-energy X-ray images). The technology mimics the workflow, dose and techniques of state-of-the-art mobile DR X-ray detectors.
“Thanks to SpectralDR, the Reveal 35C takes general X-ray to the next level because you can simultaneously get true bone and soft tissue subtracted images for any X-ray clinical task plus a high-quality DR image in one exposure at the lowest dose,” said Dr. Karim S. Karim, CTO at KA Imaging.
The spectral images provide enhanced visualization of abnormalities including indeterminate lung nodules, coronary calcium, pneumonia, tips of lines and tubes, pneumothorax, in-dwelling devices and retained surgical bodies.
For more information: www.kaimaging.com

 February 10, 2026
February 10, 2026