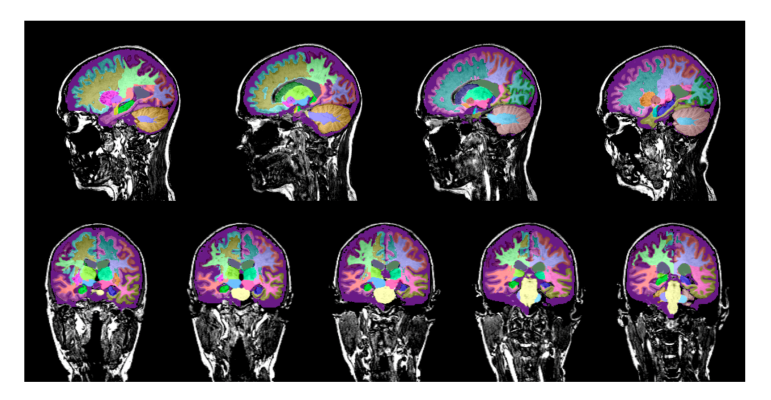
June 9, 2022 — Infinitt North America and Brainreader A/S announced today a wide ranging and unique partnership to expand and deepen the use of Brainreader's FDA cleared Neuroreader software. Neuroreader Report analyzes an MRI sequence to determine whether brain structures are abnormal and to what extent. It visualizes and quantifies 45 individual brain structures in less than ten minutes after Magnetic Resonance Imaging.
"We are very excited about the Infinitt/Brianreader partnership," said Brainreader CEO Mads Fiig. "This relationship combines forward looking science with an extremely useful tool for clinicians. Omar Rocha, our U.S. President, has been instrumental in building this wide embrace of the Infinitt customer base."
Infinitt North America, an award-winning developer of enterprise imaging solutions for healthcare, will utilize Neuroreader throughout its U.S. operations, focusing on Hippocampal lobe volume, atrophies and trends as well as applying data from Brainreader's advanced diversified normative datasets. Brainreader's expanding AI technology brings objectivity into clinical practice, detecting anomalous brain patterns, and is a proven guide for clinical decision making.
"AI and rigorously applied clinical science have resulted in new uses for brain data as a clinical tool, promoting earlier treatments, and more accurate diagnoses", said David Smarro, Infinitt N.A. President and CEO. "Neuroreader speeds up the process, increases accuracy and will improve clinical outcomes."
"Infinitt's large North American customer base provides an opportunity for both companies to uniquely contribute" said Omar Rocha, U.S. Brainreader President, "to improving doctors’ speed, accuracy, and depth of understanding as well as providing actionable aid in diagnosis and treatment."
For more information: www.infinittna.com


 February 23, 2026
February 23, 2026 









