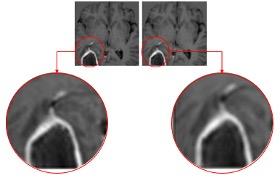
June 30, 2021 – IQ-AI subsidiary Imaging Biometrics (IB) was awarded a U.S. patent for its artificial intelligence (AI) software technology that eliminates the need for gadolinium-based contrast agents (GBCAs) in medical imaging exams. A zero-dose exam potentially offers remarkable benefits which include a more comfortable patient experience, more productive radiology departments, and reduced risks associated from the long-term, albeit uncertain, side effects of repeated GBCA use.
The fully automated AI technology, called IB Zero G, accepts non-contrast medical images as inputs and produces a synthetic image series that mimics contrast-enhanced images of comparable diagnostic quality. Currently in the investigational stage, IB Zero G is compatible with all magnetic resonance imaging (MRI) scanner platforms.
Injected intravenously, GBCAs improve diagnostic interpretation of MR imaging by highlighting abnormal areas of internal tissues and structures. Gadolinium, a heavy metal, has special paramagnetic properties which make it useful as a contrast agent. However, gadolinium can stay in a body for years, introducing potential safety and liability concerns for patients and healthcare institutions. While there are no known long-term adverse side effects from retained gadolinium in most patients, certain patients are at higher risk of adverse side effects after receiving GBCAs. IB Zero G offers an extremely attractive alternative for these patients and healthcare providers.
By eliminating the need to acquire a contrast-enhanced series, IB Zero G means less time in the scanner for patients. And for healthcare systems, IB Zero G provides increased scanner availability and a reduction in gadolinium expense.
“This patent underscores the major impact AI applications can have in healthcare,” said Michael Schmainda, CEO of IB. “IB Zero G has the potential to significantly disrupt routine clinical workflows on a global basis and help millions of patients receive higher quality and safer MR exams,” Schmainda added.
For more information: www.imagingbiometrics.com


 February 06, 2026
February 06, 2026 









