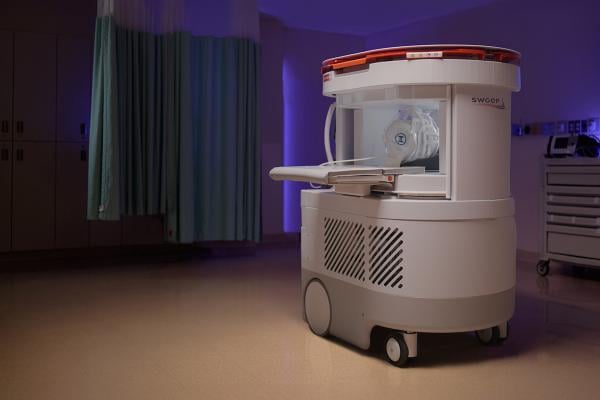
July 17, 2025 – Hyperfine, Inc.recently announced the first commercial sales of the next-generation Swoop system.The Swoop system is the first FDA-cleared AI-powered portable MRI system for the brain. The Swoop system is powered by proprietary Optive AI software.
Two pioneering hospitals in the northeast U.S. plan to utilize the technology in their intensive care units and emergency department settings.
The initial sales of the next-generation Swoop system—purchased by two top-tier hospitals in the northeastern United States—demonstrate a strong market response following FDA clearance and mark a critical step toward the widespread clinical adoption of portable brain imaging with diagnostic-level clarity.
“This marks the start of a new phase for Hyperfine — one where our vision to transform MRI access is driven by a new system that is commercially ready, is clinically valuable, has outstanding image quality and functionality, and is garnering strong customer interest,” commented Maria Sainz, president and CEO of Hyperfine. “Attaining the milestone of first commercial sales and deliveries of the new Swoop system just weeks after FDA clearance is the result of seamless execution across the organization — from industry-leading product development to accelerated clearance to rapid manufacturing ramp to initial sales. We are excited about the momentum with the new Swoop system and the growth we anticipate in the second half of 2025 and beyond.”
The new Swoop system features innovations specifically engineered to deliver the highest signal-to-noise ratio, which, when paired with the Optive AI software, achieve exceptional image quality, including improved resolution, uniformity, and faster acquisition times. This new level of image quality has the potential to dramatically drive the adoption of the Swoop system across sites of care and multiple clinical applications. The new Swoop® system also delivers a user and patient-centric design to accommodate a broad patient population—especially beneficial for pediatric, elderly, or anxious patients—making MRI more accessible for all.
For more information about the Swoop Portable MR Imaging system, please visit HyperfineMRI.com.


 February 13, 2026
February 13, 2026 









