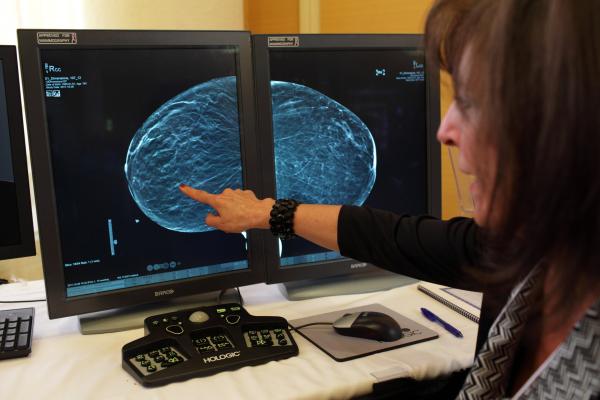
May 29, 2013 — Hologic Inc. announced that the U.S. Food and Drug Administration (FDA) approved the use of Hologic's new C-View 2-D imaging software. C-View 2-D images may now be used in place of the conventional 2-D exposure previously required as part of a Hologic 3-D breast tomosynthesis screening exam.
C-View images are generated from the 3-D tomosynthesis data acquired during the mammography exam, eliminating the need for additional 2-D exposures. The combination of Hologic's 3-D and C-View 2-D images results in less time under compression, for greater patient comfort and a lower radiation dose, while still providing the 2-D images required as part of Hologic's FDA-approved 3-D mammography screening exam.
“Large-scale clinical studies have shown that screening with Hologic's 3-D mammography technology allows radiologists to visualize the breast in greater detail than with 2-D mammography alone, which results in earlier detection of cancers while at the same time reducing the false positives associated with conventional 2D mammography that cause unnecessary anxiety and cost,” said Peter Soltani, Hologic senior vice president and general manager, breast health.
Hologic's 3-D mammography technology has been approved for use in countries recognizing the CE mark since 2008. It was approved for use in the United States for breast cancer screening and diagnosis in 2011. Hologic systems are now in use in 48 states and over 50 countries.
For more information: www.breasttomo.com


 February 18, 2026
February 18, 2026 









