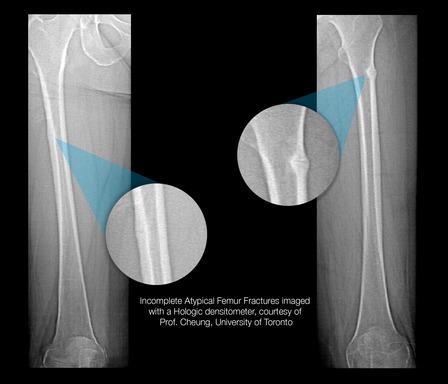
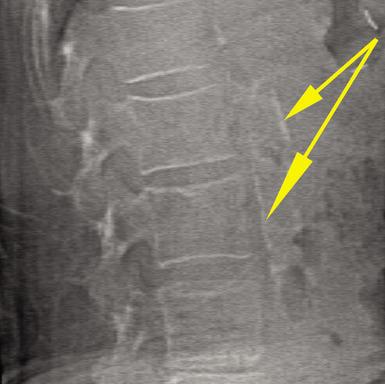
Incomplete Atypical Femur Fractures imaged with a Hologic densitometer, courtesy of Prof. Cheung, University of Toronto.
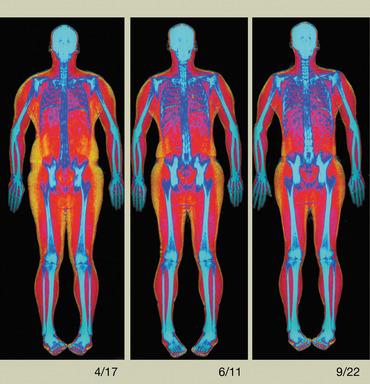
Incomplete Atypical Femur Fractures imaged with a Hologic densitometer, courtesy of Prof. Cheung, University of Toronto.
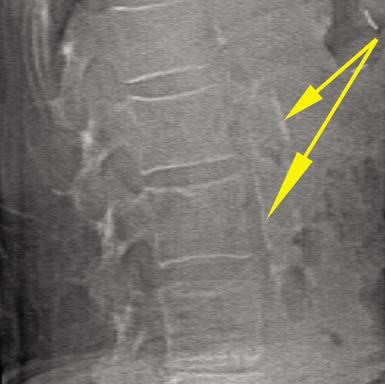
Incomplete Atypical Femur Fractures imaged with a Hologic densitometer, courtesy of Prof. Cheung, University of Toronto.
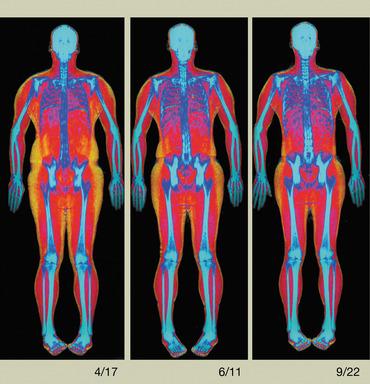
August 22, 2013 — Hologic Inc. announced the commercialization of Horizon, a redesigned DXA (dual-energy X-ray absorptiometry) imaging system for the assessment of three critical health problems — osteoporosis, cardiovascular disease and obesity. Built on Hologic proprietary technologies, the Horizon platform offers expanded technical capabilities, workflow efficiencies and improved design components to meet clinician needs. Horizon is being launched in the United States and other countries.
The Hologic Horizon DXA platform offers:
- A Single-Energy Femur Exam that allows clinicians to visualize potential atypical femur fractures, an unusual form of femur fracture which can occur as the result of bisphosphonate use;
- A FRAX 10-year Fracture Risk Report which can aid in the early detection of osteoporosis by taking into account risk factors in addition to bone density;
- High-Definition Instant Vertebral Assessment (IVA-HD) capability for significantly improved detection of vertebral fractures;
- An Abdominal Aortic Calcification feature through Hologic's IVA-HD technology, which enables clinicians to visualize abdominal aortic calcifications, a significant predictor of cardiovascular disease; and
- Advanced Body Composition assessment with visceral fat estimation to assist in the evaluation of metabolic health.
The Horizon platform is designed to offer improved precision, stability and throughput over current products. New, clinically relevant built-in enhancements include a high-resolution multi-element detector array with ceramic detector technology for improved bone mapping and image quality; a high-frequency pulsing power supply that offers greater system stability and future flexibility, and a filter drum to support future development initiatives.
"We expect the technical advancements built into our Horizon platform will set a new standard for image quality and operational efficiency," said John Jenkins, Hologic vice president of marketing for specialty imaging products. "Hologic pioneered the DXA market with the introduction of the first QDR bone densitometer, and we have maintained our leadership position through continued investment in research and development. The Horizon platform is a great example of this investment. The new system combines a wealth of proprietary, advanced technologies that provide clinicians with the most accurate diagnostic information available. We remain committed to providing technologies that will differentiate Hologic from our competitors and help women and men throughout the world lead healthier, stronger lives."
For more information: www.hologic.com


 January 22, 2026
January 22, 2026