August 23, 2023 — iCAD, Inc., a global medical technology leader providing innovative cancer detection and therapy solutions, today announced Health Canada has issued a device license for ProFound Risk version 2.0, the latest version of iCAD's personalized 1-2 year breast cancer risk assessment solution.
"We are delighted to receive the device license from Health Canada for the latest version of ProFound Risk, marking a major milestone in our mission to help women worldwide know where and when breast cancer may be hiding," said Dana Brown, President and CEO of iCAD, Inc. "This regulatory approval further validates the clinical efficacy of our technology, paving the way for its use in healthcare facilities across Canada, where breast cancer accounts for approximately 25% of new cases of cancer and 13% of all cancer deaths in Canadian women.[i] Our technology offers the potential to address this significant unmet burden by empowering clinicians to personalize care for patients at higher risk of developing breast cancer in the next 1-2 years, and ultimately improve outcomes for women."
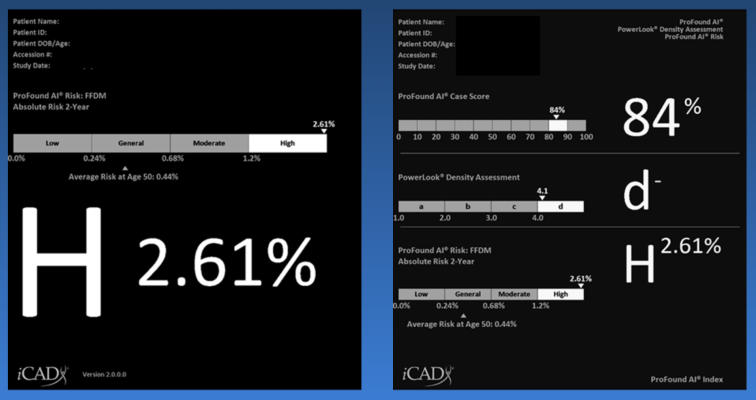
Available for both 2D and 3D mammography, ProFound Risk harnesses the power of artificial intelligence (AI) to provide personalized, accurate, and efficient risk estimations for each woman within the upcoming 1-2 years, despite having a current mammogram with no current evidence of breast cancer. By uniquely combining a range of risk factors, including age, breast density, and subtle mammographic features, this first-in-kind technology empowers clinicians to tailor screening and intervention strategies based on individual patient risk profiles, offering a more personalized and holistic approach to breast cancer detection and risk assessment.
A growing body of clinical evidence has demonstrated ProFound Risk's unique ability to more accurately predict short term cancer risk compared to commonly used risk models, including Gail and Tyrer-Cuzick. According to a recent study published in Science Translational Medicine, ProFound Risk for 3D mammography is up to 2.4 times more accurate than traditional risk models for short-term risk assessments.[ii],[iii] Another study published in the Journal of Clinical Oncology demonstrated ProFound Risk for 2D Mammography is more accurate than Tyrer-Cuzick v8, a commonly used lifestyle risk model, for both short-term and long-term risk assessments.[iv]
"We are committed to advancing women's health through cutting-edge technology and are dedicated to delivering innovative solutions that have a meaningful impact on patient care," added Ms. Brown. "The device license for ProFound Risk from Health Canada represents another giant leap forward in our journey to transform breast cancer risk assessment, and we remain focused on our mission to improve the lives of women and healthcare professionals worldwide."
For more information: www.icadmed.com
References:
[i] Government of Canada. Breast Cancer Fact Sheet. Accessed via https://www.canada.ca/en/public-health/services/chronic-diseases/cancer/breast-cancer.html.
[ii] Eriksson, M et al. A risk model for digital breast tomosynthesis to predict breast cancer and guide clinical care. Science Translational Medicine. 14 (644). 2022 May 11. Accessed via https://www.science.org/doi/10.1126/scitranslmed.abn3971
[iii] Eriksson M, Czene K, Strand F et al. Identification of Women at High Risk of Breast Cancer Who Need Supplemental Screening. Radiology. 2020 Sept 8.
[iv] Eriksson M, CzeneK , Vachon C, Conant E, Hall P. Long-Term Performance of an Image-Based Short-Term Risk Model for Breast Cancer. Journal of Clinical Oncology. DOI: 10.1200/JCO.22.01564.


 February 18, 2026
February 18, 2026 









