December 12, 2018 — Guerbet LLC USA highlighted new and next-level product offerings and partnerships in contrast media, injectors, disposables, digital solutions and services at the 2018 annual meeting of the Radiological Society of North America (RSNA), Nov. 25-30 in Chicago.
At Guerbet's booth, interactive workstations provided live demonstrations of novel clinical tools for diagnostic and interventional imaging, including:
-
Contrast & Care, a next-level contrast media injection management solution that enables imaging centers to collect, archive, examine and share data directly with Guerbet's injectors to help radiologists with patient care; and
-
Imalogix, a new self-learning neural network that enables organizations to understand and benchmark best practices from the mass learnings in real time across a network of providers to drive continuous improvement.
Guerbet also highlighted additional key milestones achieved this year, including:
-
Product launches:
-
SeQure and DraKon, two novel microcatheters for tumor and vascular aneurysm embolization procedures;
-
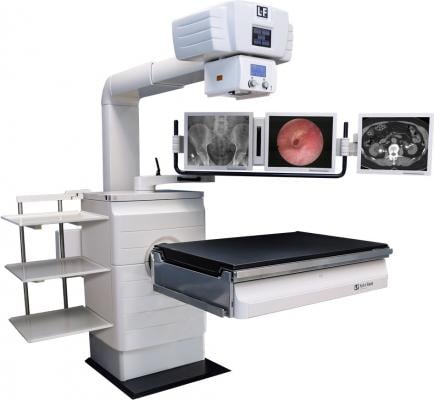
L-F Hydra Vision Digital Imaging System, a real-time digital X-ray imaging device in urological imaging
-
-
Partnership:
-
Joined forces with the Association for Medical Imaging Management (AHRA) "Defining Our Future" campaign, an initiative to support the education, development, and empowerment of new and future medical imaging professionals.
-
Guerbet's macrocyclic ionic contrast agent Dotarem (gadoterate meglumine) remains the first and only macrocyclic and ionic GBCA molecule approved by the U.S. Food and Drug Administration (FDA), according to the company, recently surpassing its 85 millionth dose worldwide. This year, Guerbet is celebrating the five-year anniversary of Dotarem's U.S. launch in March 2013 (and its first pediatric and adult injections in July and September 2013 respectively), all on the heels of Dotarem's "under age 2" pediatric approval last September.
Guerbet also celebrated the granting of the Guerbet/RSNA Research Scholar Grant, by the RSNA Research & Education Foundation to Avener Meoded, M.D., of Johns Hopkins University. The $150,000 two-year grant will help fund Meoded's research using a comprehensive "omics" approach in the study of brain neuroplasticity in pediatric arterial ischemic stroke.
For more information: www.guerbet.com


 February 20, 2026
February 20, 2026 









