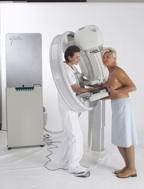
November 7, 2011 — Giotto USA yesterday announced clearance by the U.S. Food and Drug Administration (FDA) of a 510(k) application for the Giotto Image 3D and 3DL digital mammography systems. The systems, the 3D with an 18 by 24 cm bucky and the 3DL with a 24 by 30 cm bucky, may now be marketed in the U.S. The milestone was announced by Giotto USA president Robert Rusk.
“This approval means that U.S. hospitals and clinics now have a true alternative mammography system for their patients,” said Rusk. “The Giotto is the only system which has a ring-shaped, tilting gantry that allows patient-friendly, face-to-face positioning and faster and easier positioning for technologists.”
Other unique features include:
- Insight View: Giotto’s new image processing software coupled with its second-generation Selenium2 detector technology produces beautiful mammography images with greater potential to visualize small micro calcification.
- Inclined Positioning: Use of inclined positioning is widely acknowledged to increase the amount of retro mammary tissue that can be visualized. With the gantry slightly inclined, the patient can be more relaxed and the breast tends to fall forward onto the bucky. Not only is more tissue close to chest wall visualized, but also the patient is more relaxed and the examination can be much less stressful.
- Two Systems in One: The Giotto can be a multi-purpose system. By adding the optional Biopsy Digit stereotactic biopsy device, the Giotto can perform stereotactic biopsy procedures, prone or upright. This makes the Giotto Image 3D and 3DL truly two systems in one, mammography and prone stereotactic biopsy all in one unit.
See a demonstration of this new alternative mammography system at RSNA 2011 Booth # 4024 in the South Building.
For more information: www.giottousa.com


 February 17, 2026
February 17, 2026 









