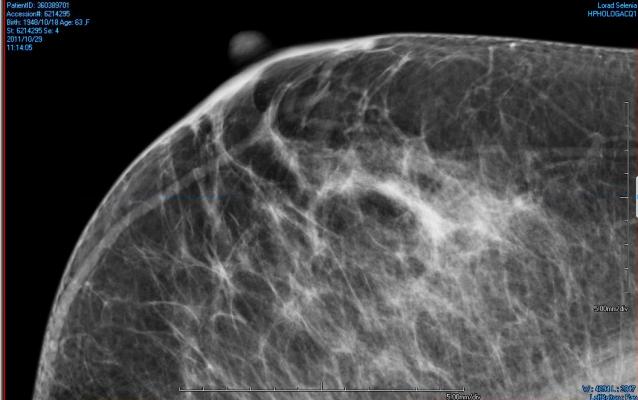
February 18, 2015 — State legislatures in Georgia and Kentucky both introduced bills last week that would require physicians to notify women about dense breast tissue and its implications for detection of breast cancer.
The Georgia bill (HB269) would require notifications to patients about breast density in all mammography, regardless of whether dense breast tissue was indicated in the results or not. Information is to be drawn from the American College of Radiology’s Breast Imaging Reporting and Data System (ACR’s BI-RADS)
The notice would require the following text:
“Dense breast tissue may hide small abnormalities. If your mammogram indicates that you have dense breast tissue, you may benefit from supplementary screening tests, including a breast ultrasound screening, a breast MRI examination, or both, depending on your individual risk factors. A report of your mammography results, including information about your breast density, has been sent to your physician’s office. If you have any questions or concerns about this report, you should contact your physician.”
In Kentucky, HB 123 would require the state Department of Public Health to provide information on its website about dense breast tissue and the implications of such mammogram results.
Thus far, 21 states have passed breast density inform laws; they are, in order from the first to the most recent: Connecticut, Texas, Virginia, New York, California, Hawaii, Maryland, Tennessee, Alabama, Nevada, Oregon, North Carolina, Pennsylvania, New Jersey, Arizona, Minnesota, Rhode Island, Massachusetts, Missouri and Ohio.
Georgia and Kentucky are among a group of seven states that have introduced a breast density inform bill in the 2015 legislative session; the others are Iowa, Mississippi, North Dakota, South Carolina and Washington.
For more information: www.itnonline.com/article/breast-density-are-you-informed


 February 18, 2026
February 18, 2026 









