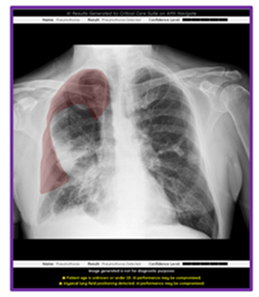
December 8, 2023 — GE HealthCare announced an industry-first US FDA 510K clearance of Critical Care Suite 2.1 featuring a Pneumothorax (PTX) algorithm for the detection, notification, triage and diagnosis of PTX. The updated PTX algorithm expands Critical Care Suite’s on-device triage capabilities by providing immediate notification of the presence or absence of pneumothorax, as well as an overlay display both on-device and in PACS to assist with PTX localization.
X-ray is the oldest form of imaging and a valuable tool on the frontlines of patient care, accounting for over 60 percent of all imaging exams.[4] Today, as technologists, radiologists, and physicians remain under tremendous pressure to manage an ever-increasing number of cases, every minute counts when dealing with high-risk procedures and critical conditions like PTX. With the high number of chest X-rays ordered as “STAT,” or immediate priority, the triaging of true STAT orders remains a challenge.[5] [6]
As the entry point to diagnostic imaging in the emergency room, inpatient bedside imaging and the intensive care unit (ICU), GE HealthCare continues to reinvent mobile X-ray to be one of the most intuitive and technologically powerful imaging tools available to help clinicians respond fast without compromising diagnostic precision.
With this latest update, Critical Care Suite 2.1 provides immediate on-device detection and triage notifications for the presence or absence of pneumothorax (PTX). When a PTX is detected, an overlay is displayed in the area where the PTX was located both on-device as well as in PACS to assist with PTX localization, as well as improve speed and accuracy of PTX diagnosis. By hosting Critical Care Suite on-device, critical insights are available at the point of care and across the entire clinical care team.
Early users of this technology see the benefit of bedside access to critical clinical information:
“The ultimate journey for an AI tool from bench to bedside is when you involve the entire clinical team including bedside Physicians, Nurse Practitioners and Radiologists”, says Dr Amit Gupta - Division Chief of Cardiothoracic Imaging, University Hospitals of Cleveland. “GE HealthCare’s innovation with Critical Care Suite to have alerts on mobile systems is helping clinicians make decisions with confidence in these critical moments.”
The algorithm operates with a high degree of accuracy – partially localizing 100% of all detected large PTXs and 96% of all detected small PTXs, while limiting false alerts (94% specificity).[7] Results from various clinical studies also showed significant user benefits when using this technology including a 57% reduction in reporting times for clinical actionable PTXs[8] and a 17.7% increase in clinician detection of small PTXs.[9]
“Artificial intelligence applications in healthcare continue to prove their value in clinical practice and on the frontlines of patient care,” shares Jyoti Gupta PhD, President & CEO of Women’s Health and X-ray for GE HealthCare. “The adoption of these digital solutions helps unlock efficiencies across the entire clinical workflow and empowers radiologists and their teams in making critical decisions with confidence in time-sensitive situations. We are excited by the paradigm shift this kind of innovation can bring in the delivery of timely and efficient patient care enabling enhanced clinical outcomes when it matters most”.
GE HealthCare’s Critical Care Suite was co-developed with UCSF's Center for Digital Health Innovation (CDHI) and the company’s AMX platform is the first mobile X-ray system in the world to offer this on-device embedded artificial intelligence for triage. In addition to the on-device AI notifications, the AI output is also sent directly to PACS via a secondary capture DICOM image to enable rapid review by the radiologist - enabling seamless integration at the point of care without the need for additional IT infrastructure. The robust AI algorithm is trained on a large, global, diverse data set that includes more than 30,000 images from multiple countries and institutions.
For more information: www.gehealthcare.com
Find more RSNA23 conference coverage here


 February 20, 2026
February 20, 2026 









