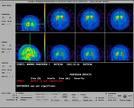
Aug 12, 2009 - The Peripheral and Central Nervous System Drugs advisory committee of the U.S. Food and Drug Administration (FDA) has voted to recommend to the FDA GE Healthcare’s DaTSCAN (Ioflupane I 123 Injection), which is used with single photon emission computed tomography (SPECT).
The panel determined DaTSCAN has a favorable risk to benefit profile, voting 11 to two with one abstention. The proposed indication for DaTSCAN is for the visualization of the dopamine transporter (DaT) distribution within the striata by SPECT imaging in patients presenting with symptoms or signs suggestive of dopaminergic neurodegeneration.
In May 2009, the FDA accepted the New Drug Application and granted DaTSCAN priority review, a designation identified for areas of unmet medical need. If approved, DaTSCAN will be the first radiopharmaceutical agent available to detect DaT distribution within the brain.
The Prescription Drug User Fee Act (PDUFA) date for DaTSCAN is September 9, 2009.
DaTSCAN has been available in Europe since 2000 and administered to nearly 1,000 patients in clinical trials.
For more information: www.gehealthcare.com


 January 27, 2026
January 27, 2026