
December 1, 2015 — An interventional radiology technique shows promise for helping morbidly obese patients lose weight, according to the preliminary results of a study being presented today at the 2015 meeting of the Radiological Society of North America (RSNA).
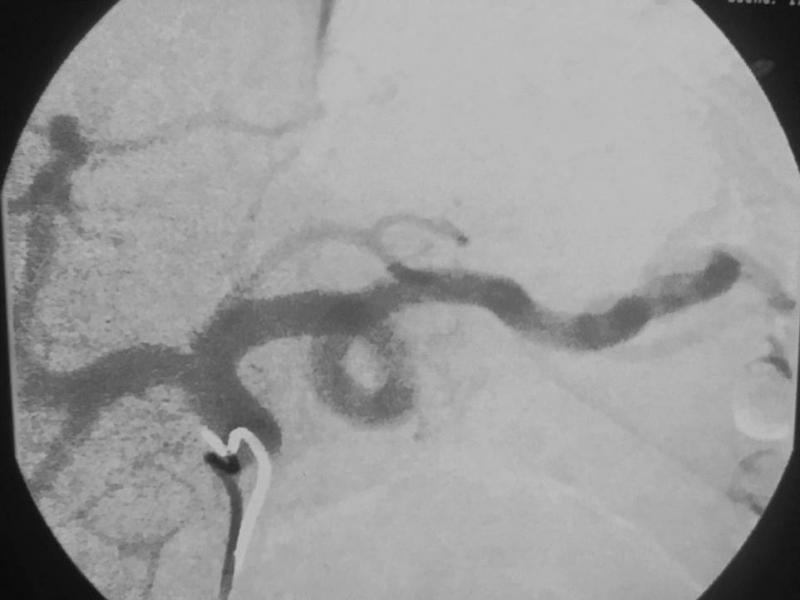
Gastric artery embolization has been done for decades by interventional radiologists as a way to stop bleeding in emergency situations, but the idea of performing the procedure as a means of treating obesity is new.
"We are showing early results that this procedure is safe and potentially efficacious for the treatment of morbid obesity," said Mubin Syed, M.D., interventional radiologist at Dayton Interventional Radiology in Dayton, Ohio.
The new approach arose from the 1999 discovery of an appetite-stimulating hormone called ghrelin that is made in the stomach. Rising levels of ghrelin are one reason that people have trouble sticking with a diet.
In order to suppress production of ghrelin, interventional radiologists embolize the gastric artery, the main artery that supplies blood to the stomach. Embolization is performed by injecting microscopic beads into the bloodstream. The beads make their way to the artery, where they block the smaller vessels. This is all accomplished through a minimally invasive, non-surgical technique through a small catheter inserted in the wrist or groin.
After reading about promising results in animal studies and preliminary research in humans, Syed set out to bring the approach to Ohio, which has some of the highest levels of obesity in the nation.
Dr. Syed was able to conduct the trial through an Investigational Device Exemption (IDE) from the U.S. Food and Drug Administration (FDA). The FDA approved the pilot study — the Gastric Artery Embolization Trial for Lessening Appetite Nonsurgically (GET LEAN) — for five morbidly obese patients with a body mass index of 40 or higher, who had failed previous attempts at weight loss through diet, exercise and behavior modification.
The initial patient in the study represented the first use of left gastric artery embolization in the Western Hemisphere to treat morbid obesity. All the patients were treated on an outpatient basis. The researchers followed a strict protocol, monitoring the patients' quality of life and taking blood samples to ensure the patients' well-being.
Three patients lost weight, including one person who, at a height of only 4 feet 11 inches, lost 50 pounds in nine months. The second and third patients experienced mild weight loss and a fourth patient, who had lost 26 pounds at her three-month follow-up, became the first diabetic to undergo the procedure.
"This is important, because diabetes is strongly associated with obesity," Syed said. "We've shown that the procedure was feasible in a diabetic patient."
While previous embolizations used the groin for access, Syed was the first to introduce the embolic agents through the radial artery in the wrist—a safer, more convenient access site in the obese population.
"In obese patients, the groin can be difficult to access," he said. "Our method is also potentially easier for patients, because they won't have to lie flat for long."
People with a previous bypass or embolization are not eligible for gastric artery embolization, and patients with recurrent depression may not benefit from the approach.
"Depressed patients are supposed to be excluded from the study because antidepressants can cause weight gain, and depressed patients often eat when they're not hungry," Syed said.
The research is still in its early stages and more results are needed before the method comes into clinical use. Gastric bypass, a procedure in which the stomach volume is surgically reduced, remains the standard treatment for morbidly obese patients who have failed other interventions.
Still, the initial results are an encouraging sign of a possible new weapon against obesity.
"We're seeing good results so far," Syed said. "We've had no major adverse events, and we hope to study more patients in the future with the same or different embolic agents."
The researchers are still in the process of selecting a fifth patient for the study.
Co-authors on the study are Kamal Morar, M.D., Azim Shaikh, M.D., M.B.A., Paul Craig, M.D., M.A., Talal Akhter, M.D., Hooman Khabiri, M.D., and Omar Khan.
For more information: RadiologyInfo.org



 February 23, 2026
February 23, 2026 









