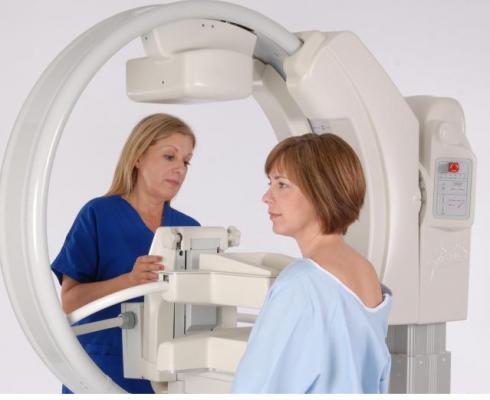
March 2, 2017 — Gamma Medica announced it has partnered with Hospital Services Limited (HSL), a Dublin, Ireland-based established medical device company that distributes, installs and services radiology capital equipment and medical devices. This partnership will provide women with dense breast tissue access to Gamma Medica’s LumaGEM Molecular Breast Imaging (MBI) system in the United Kingdom, Ireland and the Channel Islands.
Women are eligible for the United Kingdom’s National Health Service (NHS) breast screening program at age 50-79 years, with women being invited to be screened every three years. Similarly in Ireland, women 50-69 years old are eligible for breast screenings every two years. Dense breast tissue not only increases the risk of developing breast cancer, but decreases the visibility of a cancer on conventional mammograms and other forms of anatomical imaging devices. Dense breast tissue and cancer both appear white on mammograms, making it difficult to distinguish between the two. This may lead to false negatives, unwarranted biopsies or delayed diagnoses.
Ideal for dense breast tissue, MBI significantly improves early detection of breast cancer in women with dense breast tissue. The technology has proven to be as effective as a secondary screening method compared to ultrasound or magnetic resonance imaging (MRI), with far fewer false positives. Peer-reviewed clinical research reports the use of LumaGEM MBI reduces the need for tissue biopsies by 50 percent compared to other modalities. MBI is also more comfortable and better tolerated by most patients than conventional mammography or MRI.
A retrospective study, which complemented earlier published prospective clinical research, was published in the American Journal of Roentgenology’s August 2016 issue. This retrospective study monitored over 1,700 women with dense breast tissue over a three-year period. The study confirmed LumaGEM’s additional cancer detection rate of 7.7 cancers per 1,000, an increase from three cancers per 1,000 with mammography alone. The study also concluded that of the additional breast cancers found, approximately 85 percent were invasive and node negative, indicating they were detected at an early stage and therefore presented the patient with the likelihood of a better prognosis.
For more information: www.gammamedica.com


 February 18, 2026
February 18, 2026 









