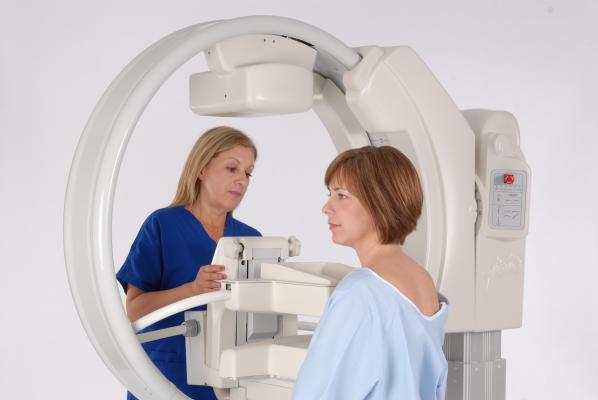
October 4, 2013 — Gamma Medica announced the updated LumaGEM Molecular Breast Imaging (MBI) system. The U.S. Food and Drug Administration (FDA)-approved system improves the operator experience and integration with hospital informatics. It has a dual head, solid-state digital imaging system that utilizes cadmium zinc telluride (CZT) technology for high precision molecular breast imaging. MBI has been shown to improve the detection of breast cancer in women with high-density breast tissue.
New for LumaGEM at RSNA 2013 is a redesigned collimator increasing sensitivy by 20 percent. This new design will accelerate image acquisition and decrease the procedural time. Additionally, the company introduces an improved breast compression system designed to maximize patient stability, helping to ensure consistent, high-quality images.
The latest evolution in a new generation of functional breast imaging, MBI technology is a high-resolution imaging system that provides a more diagnostically specific, less costly and easier-to-interpret alternative to breast MRI in many similar applications.
LumaGEM detects tumors as small as 5 mm in mammographically dense and other difficult-to-image breast tissue. The technology is distinguished from mammography by its ability to visualize tumors surrounded by non-cancerous glandular tissue, which often goes undetected on mammograms. Studies also show MBI also may be superior to mammography for imaging women with difficult-to-detect lobular tumors, scar tissue from previous surgeries, implants and small distant metastases.
“More than 40 percent of women have dense breast tissue,” says Gamma Medica president and CEO Jim Calandra. “Both physicians and patients themselves have become increasingly aware of the shortcomings of mammography in imaging these women. The result has been growing advocacy and legislation supporting the needs of women with dense breasts. MBI is an advanced technology that can make an important difference for this population.”
These and other enhancements follow a $16 million series A round of financing last summer from healthcare investment firm Psilos Group Managers and the opening of a new world headquarters in Salem, N.H., in August.


 February 18, 2026
February 18, 2026 









