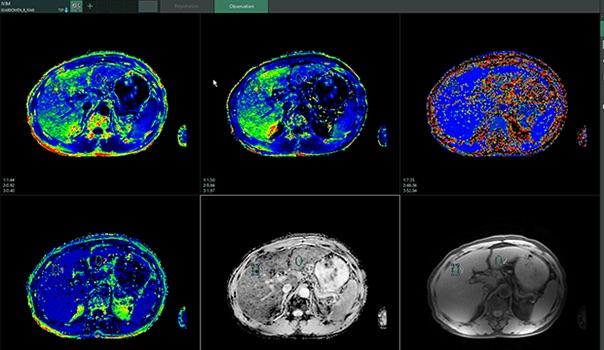
The new Intravoxel Incoherent Motion (IVIM) MR application in Synapse 3D
February 7, 2019 — Fujifilm Medical Systems U.S.A. will debut the latest version of its Synapse 3D solution at the Healthcare Information and Management Systems Society (HIMSS) global conference and exhibition, Feb. 11-15 in Orlando, Fla. The latest Synapse 3D Advanced Visualization software introduces five new applications, in addition to its current 50 clinical applications, that will be available for demonstration.
An enterprise-wide solution designed to quickly perform advanced visualization workflows and access 3-D rendered images, Synapse 3D boasts a total of 55 advanced applications, including five newly launched tools:
- Breast Analyzer MR — A multi-faceted application that analyzes breast tumors in magnetic resonance imaging (MRI) and generates Breast Imaging-Reporting and Data System (BI-RADS) reports. It includes kinetic curves for wash-in and wash-out, and subtraction of pre- and post-contrast. The tool also performs a variety of measurements including distance from the nipple, distance from the skin, distance from the chest wall and tumor volume.
- Delayed Enhancement MR — Designed with input from cardiologists, this application analyzes myocardial viability, provides delayed enhancement area and volume measurements, and displays the enhanced area overlaid in the bulls-eye map.
- Endoscopic Simulator — With simulation now a key tool in training of physicians, this application was designed specifically for laparoscopic surgery simulation. It simulates inflation of the abdomen, port placement and positioning. The tool also offers the capability to automatically segment vessels, skin, bone, pancreas, spleen and tumors.
- Intravoxel Incoherent Motion (IVIM) MR — The tool uses diffusion-weighted images to provide quantitative data from tissue microcapillary perfusion. It also automatically displays an ADC map, eADC map, D map, D* map and f map.
- Prostate Viewer MR — A robust application to support prostate cancer diagnosis, this tool analyzes prostate gland tumors and generates Prostate Imaging-Reporting and Data System (PI-RADS) reports. It measures both diameter and volume of the prostate gland and lesions. It also features an array of viewing tools for comparative observation of multiple series, T2, ADC, DWI, DCE and time intensity curves.
In addition to these new applications, Synapse 3D delivers a comprehensive suite of applications consisting of general-use and specialty options for advanced image visualization and analysis. The software features Image Intelligence recognition technology that helps optimize processing capabilities, providing increased confidence in image assessment for providers. Designed with extensive clinical input, Synapse 3D incorporates intuitive, logical workflows that guide users to efficient and accurate results that can lead to improved patient outcomes.
For more information: www.fujimed.com


 March 06, 2026
March 06, 2026 









