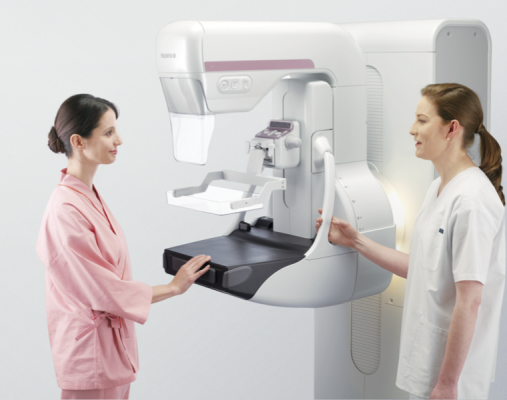
March 23, 2015 — Fujifilm Medical Systems U.S.A. Inc. showcased the Aspire Cristalle at the 25th Annual Interdisciplinary Breast Center Conference sponsored by the National Consortium of Breast Centers, March 14-18 in Las Vegas.
Fujifilm’s new imaging technology and advanced algorithms provide high-quality imaging of all breast types, including dense breasts and implants, helping to reduce the anxiety of repeat exams and higher-dose procedures. The patented Comfort Paddle is one of many features designed to make mammograms more comfortable and easier to endure for patients.
Aspire Cristalle also makes it easier for technologists to perform exams accurately and quickly. From the one-button startup, to the one-button mirrored positioning, combined with gentle, even compression and automated release features, technologists will find Aspire Cristalle easy to work with. The system combines innovative detector engineering with patient-focused ergonomics, resulting in faster, more confident diagnosis for clinicians and exceptional patient comfort.
For more information: www.fujimed.com


 February 18, 2026
February 18, 2026 









