Michael McGuire, M.D., chief of urology at NorthShore University Health System in Chicago, is an early adopter of MRI fusion targeted biopsy and sits on the Clinical Advisory Board of Focal Healthcare.
July 17, 2014 — Focal Healthcare was founded as a spin-off company out of the Centre for Imaging Technology Commercialization (CIMTEC). Focal is developing and marketing MRI (magnetic resonance imaging)-ultrasound fusion biopsy and therapy systems that have strong potential to become the new gold standard in prostate care by capitalizing on CIMTEC's intellectual property, as well as its extensive technology development and commercialization expertise in the medical imaging field.
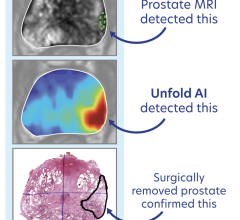
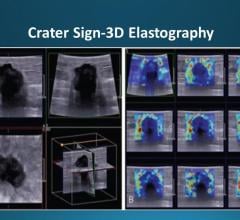
The current paradigm for prostate cancer care has remained unchanged for more than 25 years. It is well accepted that prostate cancer is fundamentally over-treated either through irradiating or surgically removing the entire prostate; both options include significant risk for life-altering side effects. This is due to the inherent limitations of ultrasound image quality, which is currently used to guide urologists in extracting random biopsy samples from the prostate. Oftentimes, this method leads to the detection of insignificant cancers or the misdiagnosis of serious cancers.
Michael McGuire, chief of urology at NorthShore University Health System in Chicago believes strongly in Focal Healthcare's products and is one of three highly respected experts in the prostate world who sits on its Clinical Advisory Board.
"I am pleased to be able to play a role in the success of Focal's game-changing prostate therapy products by testing the equipment and providing valuable feedback," McGuire said. "The superior capabilities of their products will enable urologists to accurately record biopsy locations for active surveillance of prostate cancer, as well as allow us to appropriately administer therapy, which will significantly reduce the incidence of over-treatment and improve quality of life for our patients."
Focal Healthcare has several patents, a management and development team with more than 30 years of experience in the prostate imaging and clinical device development space, access to validated underlying science and products with portability, cost effectiveness and ease of use.


 December 04, 2025
December 04, 2025