February 10, 2014 — Fluke Biomedical, a manufacturer of biomedical test instruments, acquired Unfors RaySafe.
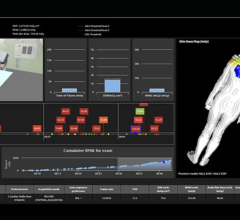
Unfors RaySafe produces quality assurance devices for diagnostic x-ray including real time dose monitoring systems for medical personnel and patient dose tracking software solutions.
Fluke Biomedical's goal with this acquisition is to provide healthcare providers, institutions and medical device manufacturers with a complete portfolio of test equipment. Both companies endeavor to provide user-friendly products with advanced technology and maximum measurement accuracy.
Fluke Biomedical manufactures biomedical test and simulation products, including electrical safety testers, patient simulators, performance analyzers and fully integrated and automated performance testing and documentation systems. With worldwide sales and distribution channels, Fluke Biomedical anticipates growth through innovative design and creative solutions for its customers.
For more information: www.flukebiomedical.com, www.raysafe.com
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now


 February 04, 2026
February 04, 2026