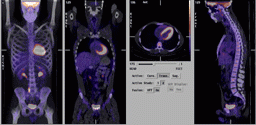
March 26, 2010 - For a patient with heart failure, checking whether the heart could benefit from bypass surgery or a stent is critical to ensuring survival. One imaging technique, positron emission tomography (PET) with the imaging agent fluorodeoxyglucose (FDG), may provide doctors with the information they need to make more informed treatment decisions, according to research published in the April issue of The Journal of Nuclear Medicine (JNM).
"This study shows a trend toward better patient outcomes when PET is used in their diagnostic work-up and has the potential to streamline management, reduce cost and improve survival in heart failure patients," said Kevin C. Allman, M.D., senior staff specialist, PET and nuclear medicine department, Royal Prince Alfred Hospital in New South Wales, Australia, and author of an invited perspective article in JNM.
The study reported in JNM is a substudy of one of the first investigations to prospectively gather evidence in a structured randomized research protocol. The benefits of using FDG PET to assess myocardial viability—to find whether heart tissue is likely to benefit from revascularization, or restoring blood flow to the heart in order to restore impaired pumping capacity—have long been observed.
Researchers in Ottawa, Canada, analyzed data from a study that used FDG PET-directed management versus standard clinical management of patients with coronary artery disease and poor left ventricular function. The report showed that FDG PET can be a useful tool for identifying patients who would benefit from bypass surgery or other procedures to improve blood flow.
Certain factors increase the chance of success. For example, the facility used in the study had an onsite cyclotron that produced a readily available supply of FDG. The setting also had a team of clinicians with expertise in the modality, which helped to facilitate communication and interaction with the doctors who applied the information to their decision-making.
"We are confident that FDG PET viability can be used to direct therapy," said Robert Beanlands, M.D., F.R.C.P.C., F.A.C.C., chief of cardiac imaging at the University of Ottawa Heart Institute, Ontario, Canada, and lead author of the study. "In cases where it is available, we recommend this course of action because it can improve patient care and patient outcomes." Beanlands also stressed the importance of clinicians knowing how to properly use nuclear imaging data.
Cardiovascular disease remains the number one cause of death in the United States. Surgery to repair damage and restore blood flow to the heart can help some patients with congestive heart failure before it is too late. Although other imaging techniques simply characterize heart tissue as being dead (scarred) or alive (viable), molecular imaging gives doctors a more complete picture of how the heart is working, including the rate of blood flow and the metabolism of the heart. Reduced blood flow could be a sign of blocked arteries; maintained metabolism means the heart tissue in this region is still viable and would benefit from restoring flow to normal. With this information, doctors should be able to better plan treatments—and heart disease patients should have better outcomes.
Authors of, "18F-FDG PET Imaging of Myocardial Viability in an Experienced Center with Access to 18F-FDG and Integration with Clinical Management Teams: The Ottawa-FIVE Substudy of the PARR 2 Trial," include Arun Abraham, Kathryn A. Williams, Ann Guo, Robert A. deKemp, Linda Garrard, Ross A. Davies, Lloyd Duchesne, Haissam Haddad, Benjamin Chow, Jean DaSilva, and Rob S.B. Beanlands for the PARR 2 Investigators from the National Cardiac PET Centre and Division of Cardiology, Cardiovascular Research Methods Centre, University of Ottawa Heart Institute, Ottawa, Ontario, Canada; and Graham Nichol of the University of Washington-Harborview Center for Prehospital Emergency Care, Seattle, Wash.
For more information: www.snm.org


 February 13, 2026
February 13, 2026 









