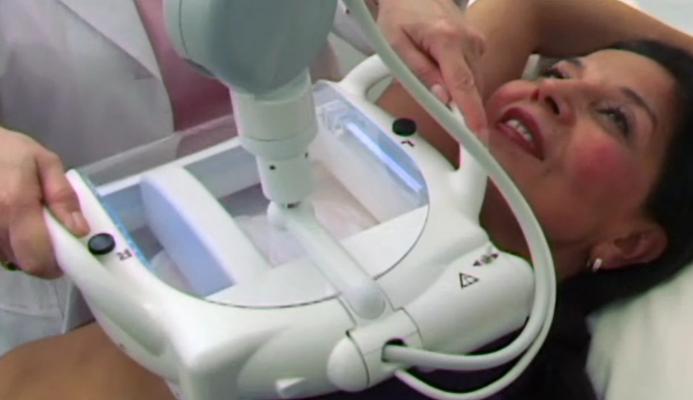
April 12, 2012 — U-Systems announced that the U.S. Food and Drug Administration’s (FDA) Radiological Devices Panel of the Medical Devices Advisory Committee recommended approval of the somo•v Automated Breast Ultrasound (ABUS) system. U-Systems’ premarket approval (PMA) application is seeking a breast cancer screening indication for the somo•v ABUS system, which is currently FDA-cleared for diagnostic use as an adjunct to mammography.
If final PMA approval is granted, the somo•v ABUS system would be the only ultrasound device in the United States approved for breast cancer screening as an adjunct to mammography for asymptomatic women with dense breast tissue. Although the FDA is not bound by the recommendations of its advisory committees, it generally follows their advice.
“With the positive FDA panel review of the somo•v for breast cancer screening, we now have the opportunity to integrate 3-D automated breast ultrasound screening into clinical practice, which will enable us to find previously undetectable cancers,” said Rachel Brem, M.D., director of breast imaging at The George Washington University Hospital, Washington, D.C. “Mammography is an effective tool at finding breast cancer, but it doesn’t work equally well in everyone. In women with dense breasts, we can’t see over a third of breast cancers, so we need other technologies, other approaches.
“The potential of 3-D ABUS in a screening environment is to find these 30 percent additional cancers that would not have been found with what is now the standard of care,” Brem added.
Dense breast tissue not only increases the risk of breast cancer up to four to six times but also makes cancer more difficult to detect via mammography, according to multiple large studies. One study, published in the New England Journal of Medicine, showed 35 percent of breast cancer goes undetected by mammography in women with dense breasts, as density masks appearance of tumors (Boyd, et al, NEJM 2007:356:227-36M). As breast density goes up, the accuracy of the mammogram goes down.
“We are very excited the FDA Panel recommended approval of a breast cancer screening indication for the somo•v ABUS system,” said Ron Ho, president and CEO of U-Systems. “If the FDA approves the somo•v PMA, this important adjunctive screening tool for women with dense breasts has the opportunity to become widely available in clinical practice. This is vitally important, because at least 40% of women in the U.S. have dense breast tissue.”
Using proprietary technology to automate the ultrasound imaging process, the somo•v ABUS system was developed specifically for the high-volume, breast cancer screening environment. The somo•VIEWer advanced 3-D workstation enables fast, accurate review and archive of patient exams, optimizing breast ultrasound screening workflow.
For more information: www.u-systems.com


 February 17, 2026
February 17, 2026