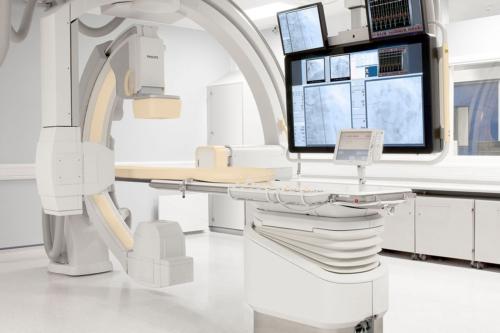
July 8, 2013 — Philips Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its AlluraClarity live image guidance system in the United States.
Philips’ AlluraClarity system with its powerful ClarityIQ technology provides high-quality imaging for a comprehensive range of clinical procedures, achieving excellent visibility at low X-ray dose levels for patients of all sizes. ClarityIQ technology will also be available as an upgrade for the majority of Philips’ installed base of monoplane and biplane interventional X-ray systems.
“All patients treated via X-ray guided interventions benefit from the advantage of low radiation exposure, but it is especially important when you are treating patients who have to undergo lengthy and complex procedures,” said Marco van Strijen, M.D., interventional radiologist at the St. Antonius Hospital Utrecht/Nieuwegein, the Netherlands. “We have been using Philips’ AlluraClarity system for more than a year now and have really grown to appreciate the low dose settings. This technology is making a difference where it really matters."
Philips’ AlluraClarity was commercially introduced outside the United States in mid-2012, and since then more than 200 systems have been ordered.
For more information: philips.us/alluraclarity


 August 14, 2025
August 14, 2025