
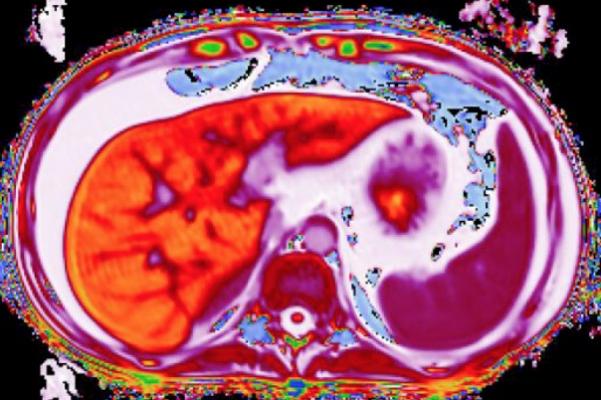
February 1, 2016 — Perspectum Diagnostics announced in November that its LiverMultiScan imaging device, which can detect early liver disease using magnetic resonance imaging (MRI), has been given 510(k) clearance from the U.S. Food and Drug Administration (FDA).
LiverMultiscan was introduced in Europe and the United States as a research device in 2014, and is now installed in leading medical centers on three continents. In addition, LiverMultiscan has applications in clinical research for therapies targeting treatment of a range of liver diseases. The technology is currently being used to assess primary endpoints in clinical trials for investigational therapies to treat non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH).
Colonel Stephen A. Harrison, M.D., FACP, FAASLD, Medical Corps USA, who is conducting these trials at San Antonio Military Medical Centre, said, "LiverMultiScan is a novel non-invasive imaging technology with the potential to dramatically change the way we approach diagnosis of liver diseases such as NAFLD. Recent published data suggest that this modality can rapidly differentiate normal liver from fibrotic NASH and then accurately predict outcomes based on the liver, inflammation, fibrosis (LIF) score. Further studies are warranted to confirm these findings, but we are excited about the possibilities this imaging modality offers for the future in NASH diagnostics."
Liver disease affects more than 15 percent of people in the United States and 10 percent of all people in the United Kingdom, and represents a significant area of unmet need in global healthcare. Obesity is a major risk factor for non-alcoholic fatty liver disease (NAFLD). The World Health Organization predicted that in 2015 the number of obese people in the world would rise to 700 million.
Used in the clinical management of patients with chronic liver disease, LiverMultiScan is the only imaging test cleared to detect early-stage liver disease. The technology offers a quantitative liver assessment in a safe, non-invasive, 15-minute MRI scan. Analysis is based on assessment of native properties of liver tissue, accurate measurement of liver fat and other metrics without the need for additional diagnostic technologies or contrast agents.
Perspectum Diagnostics also recently announced the closing of a $5 million round of private equity financing. Investors include the University of Oxford and Oxford University Hospitals NHS Trust.
LiverMultiScan utilizes quantitative MR methods, similar to recent developments in cancer and cardiac imaging, and has been shown to predict clinical outcomes for patients with liver disease. This technology provides a highly detailed map of the whole liver, making it possible to precisely identify regions of disease. This scalable technology enables as many as six patients to be tested in an hour using one MR system, with a 95-97 percent success rate. The technology can help reduce patient turnaround time and the need for retesting associated with other methods.
For more information: www.perspectum-diagnostics.com



 February 13, 2026
February 13, 2026 









