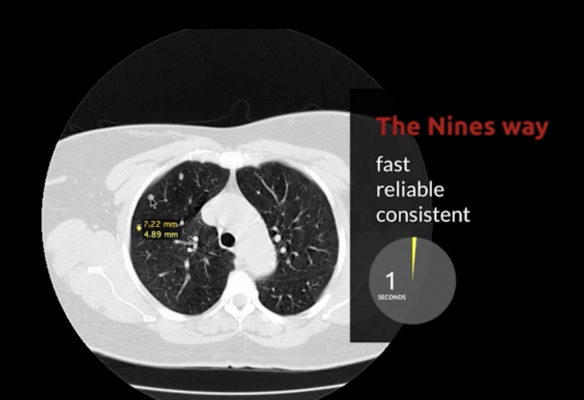
March 30, 2021 — Nines announced the 510(k) FDA clearance for NinesMeasure, an innovative lung nodule measurement tool built with artificial intelligence (AI) that can accelerate diagnoses of certain respiratory diseases.
"To our knowledge, NinesMeasure is the only lung nodule measurement tool cleared by the FDA that was developed by a combined team of radiologists and engineers collaborating every day," said Michael Kelleher, M.D., president of Nines Radiology. "This advanced tool can significantly reduce the amount of time our radiologists spend measuring pulmonary nodules, improving time to diagnosis for patients without rushing our radiologists."
The FDA clearance for NinesMeasure is the second FDA clearance in 10 months for the Silicon Valley based teleradiology practice, demonstrating the company's commitment to transforming the use of technology in radiology. Last April, Nines also received FDA clearance for artificial intelligence technology that triages mass effect conditions and intracranial hemorrhages.
Lung nodule measurement can be tedious and time consuming as each nodule has to be measured carefully to determine changes in size over time. NinesMeasure enables radiologists to quickly measure the long and short axes of selected nodules with a high level of accuracy. It can also help address inter-study consistency spanning a patient's full treatment program.
In addition to the FDA clearance for its lung nodule measurement tool, Nines also announced that its recent focus on improving clinical workflow has demonstrated a 40% gain in efficiency over three months. Nines radiologists are seeing reduced interruptions from non-diagnostic workflow automation, from one-click communications with Emergency Room physicians to an "always-ready" worklist of studies. Imaging centers and hospitals that rely on Nines can see more reliable turnaround times because radiologists are not distracted by administrative tasks and other non-diagnostic functions, which slow time to diagnosis for patients.
The radiologist-centric advances and the FDA clearances are the result of a unique innovation model and culture that the practice embodies. At Nines, radiologists are paired with in-house engineers to collaborate and develop new approaches and advanced techniques never seen in a radiology practice. For example, while most facilities typically see a tech update once a year, workflow feature enhancements occur about every three days at Nines, saving radiologists valuable time.
"In general, radiology is tech-forward in its use of digital imaging," said David Stavens, Ph.D., co-founder and CEO of Nines. "But innovation can make it better. Nines has been leading the way by pairing two seemingly disparate groups — skilled radiologists and brilliant engineers — to transform the practice of radiology to be more accessible and more efficient, delivering faster results for quality patient care. That is worth innovating."
Stavens is an entrepreneur and scientist who has pioneered several technologies and co-founded companies in Silicon Valley. He has published in the fields of machine learning, artificial intelligence and robotics. Stavens co-founded Nines in 2017 with Alexander Kagen, M.D., who is Nines' chief medical officer and serves as the site chair of Radiology at Mount Sinai West and Mount Sinai Morningside Hospitals in New York City. Kagen is also associate professor of Radiology at the Icahn School of Medicine at Mount Sinai.
For more information: www.nines.com


 February 06, 2026
February 06, 2026 









