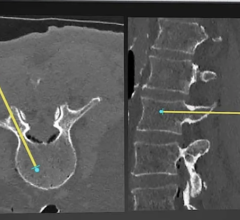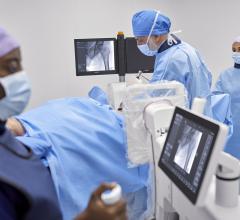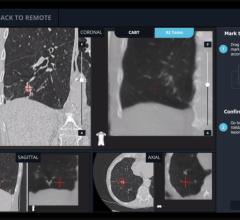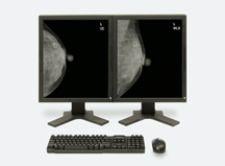
iCAD’s SecondLook Digital Computer-Aided Detection system for mammography received FDA clearance for sale with FUJIFILM’s Computed Radiography for Mammography (FCRm) systems.
The digital system coupled with the Fuji CR Mammography system will reportedly improve a user’s ability to detect potentially malignant calcifications and masses.
SecondLook Digital for FCRm is one of the first CAD products approved and available in the U.S. for use with computed radiography (CR).

 June 19, 2024
June 19, 2024