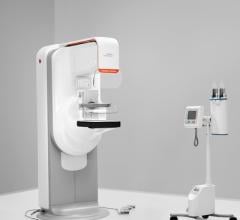
October 20, 2011 — The U.S. Food and Drug Administration (FDA) has cleared Hologic’s Trident specimen radiography system. It uses direct digital detector technology to produce high quality images for rapid verification of tissue specimens such as breast biopsy samples. Trident is a state-of-the-art, mobile system designed to reduce procedure steps, streamline workflow and give physicians increased confidence in the accuracy of their procedures.
Gary M. Levine, M.D., director of breast imaging at Hoag Breast Care, Hoag Memorial Hospital Presbyterian, Newport Beach, Calif., was one of the radiologists who evaluated the system. “Hologic’s Trident system addresses many market needs by providing exceptionally high image quality, rapid verification of tissue samples and extreme ease of use,” Levine said. “Trident is a valuable tool in helping to improve the delivery of breast care for women.”
Frequently, breast specimen imaging is performed directly on the facility’s mammography system. This takes away from the time the system could be used for normal screening and diagnostic exams. In addition, if the clinician leaves the procedure to confirm the specimen image, the patient often needs to stay under anesthesia and/or in compression during this time.
Hologic’s system is designed for use in the operating room where breast tissue is surgically excised, and in biopsy suites where minimally invasive, stereotactic or ultrasound breast biopsies are performed.
The new system is self-contained and eliminates the need for samples to be taken to the radiology department for X-ray imaging. Performing the verification in the same room as or within close proximity from the procedure improves workflow, thus reducing procedure time for the patient.
The selenium-based detector, with its 12 x 14 cm active imaging area, provides high quality imaging for the majority of breast specimens. The system’s user-friendly interface facilitates seamless workflow without the need for extensive training or experience. For even greater efficiency, the system offers one-button Automatic Exposure Control and one button export to picture archiving and communication system (PACS) or to the Hologic SecurView diagnostic workstation.
For more information: www.hologic.com


 March 03, 2026
March 03, 2026