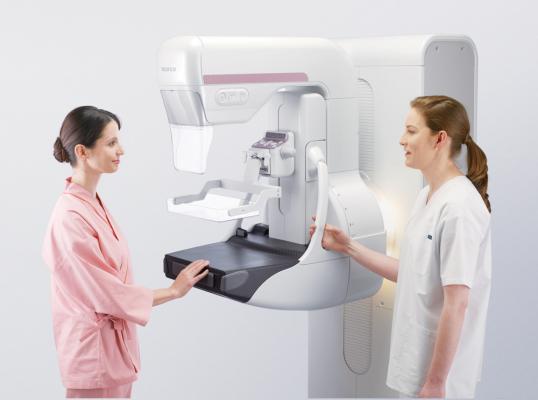
July 2, 2014 — Fujifilm Medical Systems U.S.A. Inc. announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) of the Aspire Cristalle mammography system. The Aspire Cristalle combines innovative detector engineering with well thought out patient focused ergonomics, resulting in faster more confident diagnosis for clinicians and exceptional patient comfort. First commercialized in Asia and Europe, the Aspire Cristalle has taken hold of customers' imagination and challenged the status quo in breast imaging technology across the globe.
The Aspire Cristalle brings an innovative solution to breast imaging customers around the world. New advancements include:
- Patient experience enhancements, such as the patented Comfort Paddle, are designed to make the most uncomfortable part of mammograms, more comfortable. The paddle’s soft edges, flexible composition and four-way pivot contours to the individual shape of the breast to more comfortably apply compression for optimal tissue separation. The system also features new patient grip handles and padding for added stability and comfort, even soft backlighting and graphic mural decals to help ease patient anxiety and improved access for wheelchair exams, just to name a few.
- Hexagonal Close Pattern (HCP) is a detector pixel design, engineered to deliver higher acquisition efficiency, for finer detail, higher DQE, higher MTF and lower dose than conventional square pixel arrays.
- The system’s image quality is further enhanced with Fujifilm’s image processing, iAEC automated exposure controls and ISC precisely tuned contrast, to intelligently adapt to the specific characteristics for every breast type and to image implants with ease.
For more information: aspire.fujimed.com


 February 18, 2026
February 18, 2026 









