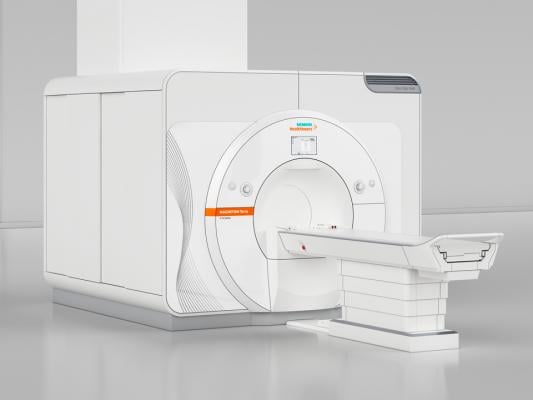

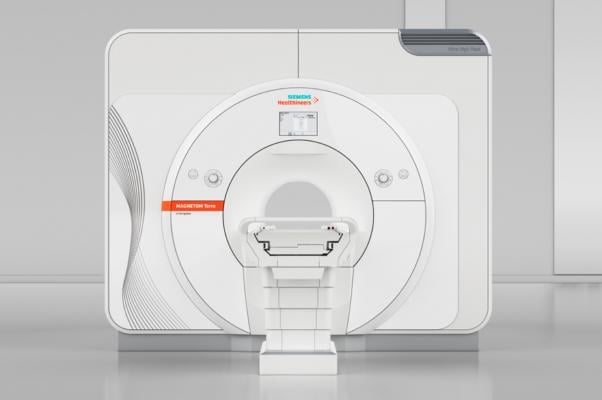
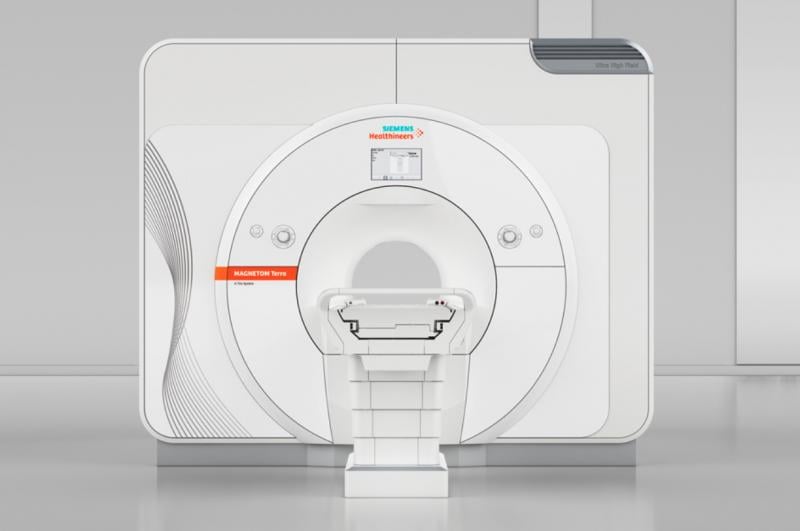
October 12, 2017 — The U.S. Food and Drug Administration (FDA) cleared the first 7 Tesla (7T) magnetic resonance imaging (MRI) device, more than doubling the static magnetic field strength available for use in the United States. The Magnetom Terra from Siemens Healthineers is the first 7T MRI system cleared for clinical use in the United States.
“The overall image quality of MRI improves with higher magnetic field strength,” said Robert Ochs, Ph.D., director of the Division of Radiological Health in the FDA’s Center for Devices and Radiological Health. “The added field strength allows for better visualization of smaller structures and subtle pathologies that may improve disease diagnosis.”
MRI scanners use strong magnetic fields and radio waves (radiofrequency energy) to generate images. The signal comes mainly from the protons in fat and water molecules in the body. When interpreted by a trained physician, images from an MRI scan provide information that may be useful in determining a diagnosis. MRI scanners come in different magnet field strengths measured in tesla or “T.” Before today’s clearance, clinical MRI systems were available in field strengths of 3T and below.
The FDA reviewed the Magnetom Terra through the 510(k) premarket clearance pathway. The FDA based its clearance on comparison to a predicate device and acquisition of sample clinical images. The agency reviewed the safety of the radiofrequency subsystem through computational modeling, simulations and rigorous experimental validation. The manufacturer also provided data from a comparative study of 35 healthy patients that compared images of the patients using the 7T device and images using the 3T device. Board-certified radiologists reviewed the images and confirmed that the images acquired on the 7T device were of diagnostic quality and, in some cases, an improvement over imaging at the 3T.
This advanced ultra-high-field scanner produces cross-sectional images of the head and knee, and is intended for patients over 66 lbs. The system’s neurological and musculoskeletal (MSK) applications have the potential to help physicians achieve unprecedented breakthroughs in clinical care by revealing functional and anatomical details not visible at lower magnet field strengths.
The ultra-high-field scanner is indicated for patients who weigh more than 66 pounds and is limited to examinations of the head, arms and legs (extremities).
Siemens said the system’s neurological and musculoskeletal (MSK) applications have the potential to help physicians achieve unprecedented breakthroughs in clinical care by revealing functional and anatomical details not visible at lower magnet field strengths.
The system has a unique dual mode feature, which permits the user to switch for the first time ever between an investigational “research mode” and a 510(k)-cleared “clinical mode” for imaging, while keeping research data and clinical images safe on separate databases, respectively. The scanner delivers superior images with up to 64 receive channels and more than twice the signal-to-noise (SNR) ratio of 3T MRI in optimized 7T neuro and MSK clinical applications. Its 80/200 gradients provide high levels of power to not only perform diffusion MRI and functional MRI (fMRI), but also to utilize the company’s Simultaneous Multi-Slice (SMS) application to accelerate advanced neurological applications for clinical routine. Its ultrafine 0.2 mm in-plane anatomical resolution potentially enables visualization of previously unseen anatomical structures.
For example, cerebral cortex imaging at 0.2 mm in-plane resolution may yield never-before-visible clinical details in cortical structure, Siemens said. The scanner’s submillimeter BOLD fMRI contrast increases linearly with field strength, which may translate in clinical use to higher resolution in neuro imaging compared to 3T applications.
The scanner has two coils optimized for clinical neuro and knee imaging. The MRI scanner leverages the syngo MR E11 software platform from Siemens Healthineers, enabling users to work in the same manner as they would with the company’s 1.5T and 3T technology. The Magnetom Terra also features the hyper-fast image reconstruction technology of the MaRS (Measurement and Reconstruction System) computer, for speeds up to 20 times faster than previous generations of 7T research scanners.
Siemens said the system has the ability to expand research capabilities, giving research centers a competitive edge and to support reputations as a key opinion leader. Currently, the vendor said more than 65 percent of 7T MRI scanners worldwide are from Siemens. In the United States, six hospitals have installed Siemens 7T systems for research purposes
Siemens said the 7T system is designed for fast installation in clinical environments and will enable reduced operating costs thanks to design that has zero helium boil-off.
“With the Magnetom Terra 7T scanner, Siemens Healthineers proudly introduces the first MRI field strength above 3T to be cleared for clinical imaging in nearly 20 years,” said Christoph Zindel, M.D., senior vice president and head of magnetic resonance at Siemens Healthineers. “Armed with the Magnetom Terra’s ultra-high-field strength, clinicians may be able to achieve new, unforeseen levels of patient care and clinical advancements through improved visualization of a wide variety of neurological disease states.”
For more information: https://usa.healthcare.siemens.com/magnetic-resonance-imaging/7t-mri-scanner/magnetom-terra/features#02161187_EN_US
Related MRI Systems content:
Watch the VIDEO Magnetic Resonance Imaging (MRI) Technology Report from RSNA 2016
Recent Advances in MRI Technology
MRI Systems Comparison Chart (will need to create a free login)
GE Healthcare Partners With Tesla Engineering to Produce Ultra High-Field 7T MRI Systems
University of Iowa Selects GE 7T MRI to collaborate on Research into Brain Disorders



 March 06, 2026
March 06, 2026 









