March 15, 2018 – CereMetrix, a subsidiary of CereHealth Corp., has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its neuroimaging analytics and quantification platform CereMetrix Silver. Providers will now have access to objective neuroimaging that detects, quantifies and analyzes brain function down to the voxel level.
The CereMetrix Silver image viewer is available to analyze brain imaging, including PET, SPECT, MRI and CT scans. The platform is designed to increase productivity and accuracy by providing reading physicians with significant workflow efficiencies, including automated cluster analysis that allows for quantitative and statistical analysis of SPECT brain scans by comparing to other registered SPECT brain scans. The proprietary workflow also features an automated reporting capabilities.
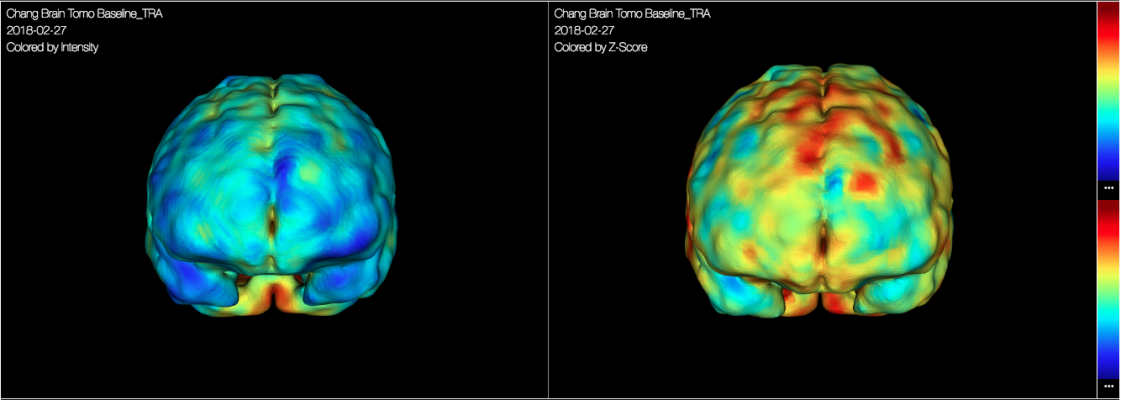
With CereMetrix Silver, reading physicians can now allow for the localization and definition of brain function and differentiate between hyper (more than average) and hypo (less than average) function in the brain. Within viewports, the reading doctor can process and display the brain image data in traditional two-dimensional formats, while simultaneously displaying pseudo three-dimensional renderings.
“With the current technology, functional brain imaging results are blended across entire anatomical areas, allowing far more subjectivity in the physician’s interpretation and findings,” said Shane Quint, Chief Technology Officer of CereScan. “CereMetrix Silver provides voxel-level measurements to help physicians objectively analyze the imaging, and make faster diagnostic decisions without compromising value-based care.”
CereMetrix Silver is to be used by trained medical professionals as a tool to aid in the evaluation and information management of digital medical images. The neuroimaging platform will also aid in the assessment of qSPECT (quantitative Single-Photon Emission Computed Tomography) brain scans. The brain imaging data, once reconstructed, will be mapped to a common brain template and anatomical map before applying a clustering algorithm to group pixels/voxels of tissue with deviations from average into clusters.
For more information: http://ceremetrix.io

 February 26, 2026
February 26, 2026