December 21, 2018 — Insightec announced that the U.S. Food and Drug Administration (FDA) has approved an expansion of the indication of Exablate Neuro to include the treatment of patients with tremor-dominant Parkinson's disease (PD).
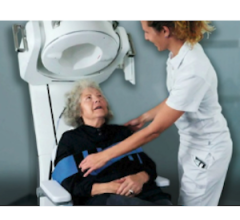
The Exablate Neuro is a focused ultrasound device for performing incisionless thalamotomy guided by magnetic resonance imaging (MRI). This expansion adds medication-refractory tremor from PD to the current Exablate Neuro indication for incisionless, focused ultrasound thalamotomy for medication-refractory essential tremor.
"This is another validation of a great technology," said Jeff Elias, M.D., of the University of Virginia School of Medicine. "Patients are attracted to the less invasive aspects of focused ultrasound. Now Parkinson's patients, for whom tremor is their primary disability, have more treatment options than conventional cranial surgery. While focused ultrasound is not curative for Parkinson's disease, it can provide significant quality of life benefits. Research continues for the other symptoms of Parkinson's," he concluded.
Tremor, rigidity, slowness of movement and postural instability are the cardinal features of Parkinson's disease (PD). In an estimated 10 - 20 percent of PD patients, the primary symptom is tremor. These patients initially have tremor and as the disease progresses, they may experience an onset of relatively mild bradykinesia and rigidity. Upon further disease progression, tremor remains the symptom with the most severe impact on daily activities.
During the focused ultrasound treatment, sound energy passes safely through a patient's skull, with no surgical incisions, to heat and precisely ablate the target in the thalamus. The treatment allows the neurosurgeon to create a personalized treatment plan and to evaluate feedback of the patient's symptom relief or potential side effects in real-time. Patients must be at least 30 years of age.
This approval was based on data from a study led at the University of Virginia School of Medicine and was also conducted at Swedish Neuroscience Institute in Seattle.
For more information: www.insightec.com


 March 03, 2026
March 03, 2026