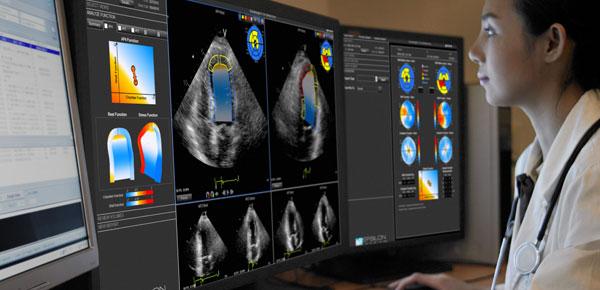
July 30, 2013 — Epsilon Imaging Inc. has received the CE mark for its EchoInsight visualization and analysis platform with practical strain imaging for improved quality, standardization and workflow in echocardiography interpretation. The CE mark will allow EchoInsight to be sold in the European Union.
By assisting clinicians to quickly and easily integrate strain imaging into the clinical environment, EchoInsight enables improved analysis and interpretation of studies. EchoInsight provides immediate access to analysis, intuitive features and clinically specific applications with straightforward information to improve confidence in function assessment based on study type.
The platform offers customized integration to customer healthcare information technology workflow as a DICOM compliant platform, along with remote customer support.
EchoInsight’s platform standard features include automated processing, rapid review and study comparison, endocardial border tracing, intuitive visualization and analysis, click-of-a-button wall motion assessment based on recognized standards, automatic calculation of cardiac function measurements and concise, visual-based reporting with picture archive and communications system (PACS) archival ability.
Initial customized clinical applications include:
- EchoInsight for Stress Echo with features including rapid rest-stress study comparison, and global and regional wall and chamber function analysis based on strain and ejection fraction (EF).
- EchoInsight for Cardio Oncology with features including rapid serial study comparison, and global and regional longitudinal strain and EF trending with percent change from baseline.
For more information: www.epsilon-imaging.com


 February 13, 2026
February 13, 2026 









