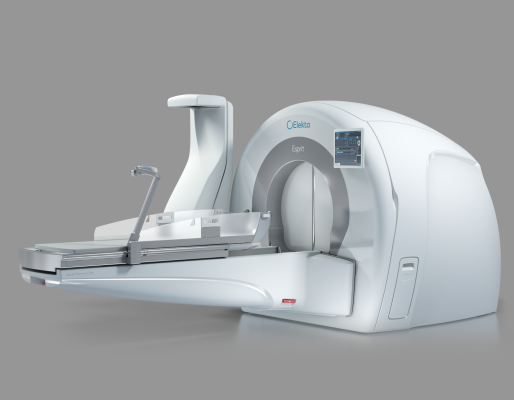
November 8, 2022 — Elekta announced that Elekta Esprit, a new Leksell Gamma Knife radiosurgery platform, received FDA 510(k) clearance. This milestone makes the system available to clinicians and people with brain disease in the U.S., as well as opening the door to other countries where FDA approval is recognized.
Leksell Gamma Knife has been designed as a gentler alternative to open surgery and conventional radiotherapy. It can target the smallest and most challenging intracranial tumors and lesions with minimal effect on healthy tissue. This vital precision safeguards motor, sensory and neurocognitive function to help protect the mind and the person.
Verena Schiller, President of Elekta’s Neuroscience Solutions, says: “It’s very inspiring to hear how patients treated with Gamma Knife are able to maintain their quality of life and go back to what they love doing after radiosurgery. Thanks to the very low dose delivered outside the target, it’s able to protect surrounding healthy tissue. Esprit takes Gamma Knife to the next level with improvements in both the patient and user experience."
With a variety of innovations now available in a single platform, Esprit continues to offer clinicians the option of frameless or frame-based workflows with a frame that provides superior visualization. Remote accessibility and collaboration tools for the treatment team are designed to meet the needs of changing environments. Esprit provides the accuracy to treat even the most challenging targets while delivering a gentler more personalized approach to radiosurgery.
Leksell Gamma Knife has over 50 years of clinical evidence and remains the gold standard of intracranial radiosurgery. The U.S. has the largest installed base of Gamma Knife systems, whose users have contributed to driving the standard of care forward. Continuous advances in its design have resulted in extremely fast automated treatment planning for clinicians, and Esprit is ideal for safely treating a broad range of indications, including multiple metastases, arteriovenous malformations, trigeminal neuralgia and vestibular schwannoma.
For more information: www.elekta.com/elekta-esprit
*Elekta Esprit is CE marked and FDA 510(k) cleared, with limited global availability.


 February 04, 2026
February 04, 2026 









