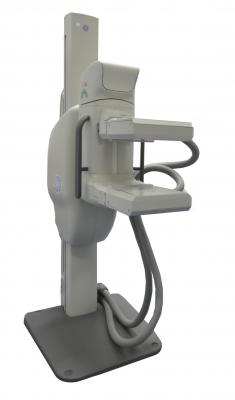
November 28, 2016 — Dilon Diagnostics and GE Healthcare announced the U.S. Food and Drug Administration (FDA) clearance of the Molecular Breast Imaging (MBI) localization accessory for breast biopsy. The Discovery NM750b and the breast biopsy accessory will be presented at the 2016 Radiological Society of North America (RSNA) conference, Nov. 27-Dec. 1 in Chicago. The Discovery NM750b will be at the Dilon booth number 6132.
The approval comes shortly after Dilon and GE signed an exclusive agreement for Dilon to distribute GE's Discovery NM750b Molecular Breast Imaging system in North America.
The Discovery NM 750b breast imaging system, designed to measure and image the distribution of selected single photon emission radioisotopes in the human body to aid in the evaluation of lesions, offers clinicians an important tool for imaging of a broad range of patients, including those with dense breasts. The MBI localization accessory is a complementary technology to the Discovery NM750b system that is designed to accurately locate, in three dimensions, lesions in the breast using information derived from stereotactic pairs of two-dimensional images. It is intended to provide guidance for interventional purposes such as biopsy and pre-surgical.
For more information: www.dilon.com, www.gehealthcare.com


 March 06, 2026
March 06, 2026 









