June 30, 2020 — DiA Imaging Analysis, a leading provider of advanced AI-based solutions for ultrasound analysis, announced today that it has received clearance from the U.S. Food and Drug Administration (FDA) 510(k) and a European CE mark for two additional AI-powered ultrasound analysis solutions.
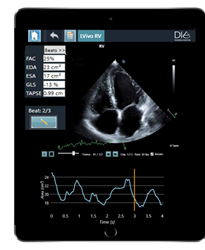
- LVivo RV is the first AI-based fully automated analysis of the heart’s right ventricle (RV) and can be used to diagnose and monitor right heart ailments in acute and chronic patients suspected to have RV injury, including those with COVID-19.
- LVivo Bladder uses AI to deliver automated bladder volume measurements on ultrasound devices, significantly reducing patient scanning time and risk of infection.
Both solutions join DiA’s LVivo Toolbox, of now six FDA/CE-cleared AI-powered ultrasound solutions in total. The company was recently awarded a COVID-19-specific government grant from Israel’s Innovation Authority to accelerate access to its AI-based offerings in response to the COVID-19 pandemic.
“Physicians now face a host of new challenges and restrictions on the frontlines that underscores the critical role of ultrasound and value of having access to AI-powered solutions,” said Hila Goldman-Aslan, DiA’s CEO and Co-Founder. “With today’s FDA and CE approvals, DiA takes a big step toward strengthening our AI offerings for cardiac ultrasound while realizing our vision of making ultrasound analysis smarter and more accessible across a broader range of healthcare segments.”
There is growing evidence linking COVID-19 mortality and right ventricle heart failure. A study at Mount Sinai Morningside Hospital found that 31% of the patients hospitalized with COVID-19 had right ventricle failure, and that 41% of patients in this subset who died had signs of RV dilation or enlargement. By automatically providing quantified 2D analysis of the RV’s size and function, LVivo RV helps detect and monitor right heart failure. LVivo RV utilizes DiA’s flagship LVivo Artificial Intelligence platform to deliver faster, more accurate analysis while at the same time enabling clinicians to shorten patient scanning and evaluation time.
“The RV has always been very difficult to evaluate, due to its unique structure and location,” said Anthony M. Demaria, M.D., a leading cardiologist at University of California, San Diego (UCSD) Medical Center and a co-investigator of the multi-center study for LVivo RV. “This is further compounded by the complexity of the analysis itself, which relies on a combination of manual calculations and visual user input. LVivo RV is a welcome and very useful addition to clinicians’ toolbox for more quickly and effectively monitoring the right ventricle.”
Additional co-investigators of the study included David Rosenmann, M.D., and Shemi Carasso, M.D., at Israel’s Shaare Zedek Medical Center and Baruch Padeh Medical Center, respectively.
With LVivo Bladder, DiA extends its expertise beyond cardiology to abdominal ultrasound. Healthcare-associated urinary tract infections (UTIs) account for up to 40% of infections in hospitals and 23% of infections in the intensive care unit (ICU). LVivo Bladder enables ultrasound users to automatically and accurately measure bladder volume, which is currently done on cart-based systems manually or by dedicated bladder scanners. LVivo Bladder’s automated volume calculation turns any ultrasound device into a bladder scanner, minimizing patient scanning time and helping to prevent unnecessary catheterization to reduce the risk of infection.
For more information: www.dia-analysis.com


 February 13, 2026
February 13, 2026 









