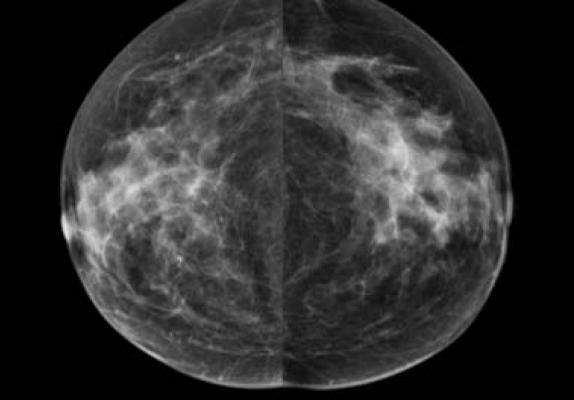
April 13, 2018 — Densitas Inc. has received U.S. Food and Drug Administration (FDA) clearance for its DENSITAS|density automated breast density software. The software analyzes the same processed digital mammograms that radiologists view and are routinely stored in picture archiving and communication systems (PACS).
The zero-click software provides radiologists with breast density assessments at point-of-care that are as accurate as the visual assessments made by experienced Mammography Quality Standards Act (MQSA)-qualified radiologists. The automated reports enhance confidence in follow-up care decisions and facilitate uniformity in density measurements across clinics and major hospital networks. The company said DENSITAS|density integrates seamlessly with PACS and enhances workflow.
Densitas has the advantage of generating breast density assessments from standard processed breast images and their priors that are stored in PACS. This provides a practical solution for integrating breast density into risk-based models in population-based screening.
DENSITAS|density is cleared for clinical use in the U.S., Europe, Canada and Australia, and is the first of several follow-on products. The software is being showcased at the 2018 Society for Breast Imaging (SBI)/American College of Radiology (ACR) Breast Imaging Symposium, April 12-15, in Las Vegas.
For more information: www.densitas.health


 January 22, 2026
January 22, 2026