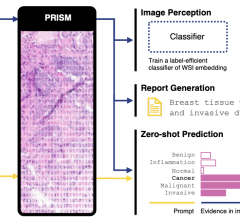
December 16, 2010 – Bracco Imaging demonstrated how its customized evidence-based diagnostic imaging solutions are meeting the demands of an evolving radiology market at the Radiological Society of North America (RSNA) meeting. The company is also showing regulatory progress related to its contrast media in computed tomography (CT), magnetic resonance imaging (MRI), nuclear medicine, cardiac catheterization, interventional/diagnostic radiology and its contrast delivery systems.
“A number of factors are shaping the evolution of radiology today, including rapid innovation, cost control and an acute awareness of product safety,” said Carlo Medici, head of global commercial operations of Bracco Imaging and president and CEO, Bracco Diagnostics.
The company’s Isovue (iopamidol injection) is available in a convenient range of iodine concentrations and packaging options, making it the top osmolar contrast media agent in the United States.
The company also continues to see robust growth stemming from its 2008 acquisition of E-Z-EM, one of the most recognized brands among radiologists and gastrointestinal (GI) physicians. Among these are the company’s injector and data management systems, which enable radiologists, administrators and technologists to gain workflow efficiencies, imaging optimization and patient safety benefits. Also, through this acquisition, Bracco is now the only provider of barium-based oral imaging products.
Safety, Efficacy and Efficiency in MRI
The company’s gadolinium-based contrast agents (GBCAs) - MultiHance (gadobenate dimeglumine) and ProHance (gadoteridol) - are increasingly the MRI agents of choice by radiologists in more than 2,000 healthcare facilities in the United States.
MultiHance was the first and only contrast agent approved for MRI of the central nervous system (CNS) in the United States, with twice the relaxivity compared to other tested extracellular contrast agents. This means greater contrast-to-noise and lesion-to-brain ratio, which may lead to improved visualization. ProHance is the only macrocyclic gadolinium contrast agent available in the United States, with an exceptionally stable Gd chelate.
Nephrogenic systemic fibrosis (NSF), a rare and potentially life threatening syndrome in patients with renal impairment, has been associated with GBCAs. This has fueled significant clinical and scientific activity in attempts to determine the etiology of this disease. In response the U.S. Food and Drug Administration (FDA) recently published new guidelines defining specific classes of GBCAs based on risk of causing NSF. In a briefing document dated December 8, 2009, the FDA wrote: “Of the 5 GBCs considered, the higher risk is associated with Omniscan, Magnevist, and OptiMARK while the lowest risk is associated with ProHance and MultiHance.”
Further, the American College of Radiology that radiologists should administer GBCAs to renally impaired patients only where necessary. The ACR also advised using the lowest possible dose.
Studies demonstrate that MultiHance provides significantly greater diagnostic information and lesion visualization when compared to two other commonly used contrast agents used at an equivalent dose. It is also FDA-approved for intravenous use in MRI of the CNS in children over two years of age.
For more information: usa.braccoimaging.com


 January 27, 2026
January 27, 2026