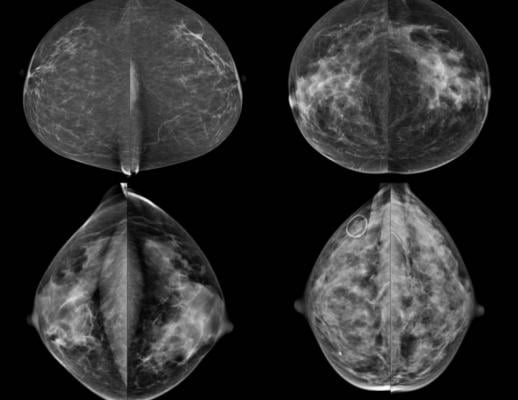
April 6, 2017 — Colorado Gov. John Hickenlooper signed the Dense Breast Tissue Reporting Bill (SB17-142) on April 6, adding to the growing list of density reporting laws across the country. The signing makes Colorado the 29th state to enact some form of legislation requiring mammography facilities to notify patients about the impact of fibroglandular breast density on cancer detection and risk.
The language of SB17-142 requires mammography providers to include a notification in the report provided to the patient if the patient is found to have dense fibroglandular breast tissue. The notice must also inform the patient that while common, dense breast tissue can make it more difficult to evaluate the results of the mammogram and it is associated with an increased risk of breast cancer.
The bill was introduced in the Colorado Legislature by Sen. Angela Williams and sponsored in the House by Rep. Jessie Danielson. The Colorado Breast Density Coalition actively advocated for the law.
“This is a victory for Colorado women,” said Williams. "Now all women in Colorado will have the same information as their doctors."
Danielson added, "This law encourages patients to discuss with healthcare providers screening options that are personalized to a patient with dense breast tissue. It is one solution to increase the early detection of breast cancer."
The law is effective on Oct. 1, 2017. Currently, New Mexico, Iowa and Kentucky have breast density reporting bills awaiting governors' action.
For more information: www.areyoudense.org


 February 18, 2026
February 18, 2026 









