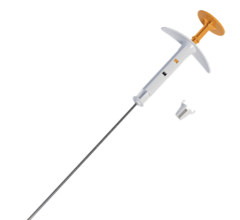
March 24, 2009 - Cianna Medical said today that the U.S. Patent and Trademark Office issued two patents that are directly related to its SAVI multi-catheter brachytherapy device for the treatment of breast cancer.
The patents, Nos. 7,497,819 and 7,497,820 and titled “Expandable Brachytherapy Device,” represent the first patents issued for a family of applications focused in brachytherapy.
The SAVI multi-catheter open architecture design allows for the delivery of patient-specific radiation that minimizes the radiation dose to healthy tissue. Published reports and clinical experience show that most women who are excluded from balloon brachytherapy because of the location of the lesion or breast size can receive treatment with the SAVI delivery system.
Cianna received 510(k) clearance for SAVI from the U.S. Food and Drug Administration in August 2007 for use during brachytherapy procedures. For appropriate patients, SAVI is an advanced technology that may reduce treatment time to just five days, versus six to eight weeks for traditional radiation therapy.
For more information: www.CiannaMedical.com


 July 11, 2024
July 11, 2024