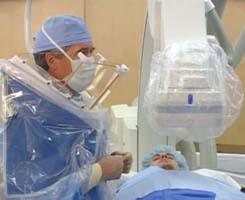
To prevent physician fatigue and back pain associated with wearing protective lead aprons in the cath lab, the ZeroGravity Radiation Protection System offers increased radiation protection without weighing down the user. Using a ceiling suspended gantry system, no weight is placed on the body of the interventionalist and it allows for smooth movement along the X, Y, Z axis. The lead apron has 1 mm lead equivalency protection from the thyroid to groin. It offers twice the protection compared to standard lead aprons. It also provides 0.5 mm protection where previously there was none available and it has front and side protection from direct and scatter radiation. The user can freely engage and disengage without assistance and without breaking sterility. Contact between the device and the body is minimal, giving an open, ventilated feeling.
CFI Medical Solutions | www.zgrav.com


 July 25, 2024
July 25, 2024 









