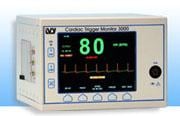
October 8, 2010 — The Ivy Model 3000 Series Cardiac Trigger Monitor offers precision image-triggering nuclear medicine, computed tomography (CT) and magnetic resonance imaging (MRI) systems, with more than 16,000 different variations in use worldwide. Ivy Biomedical Systems’ customers include GE Healthcare, Siemens Medical Systems, Philips Medical Systems, Toshiba, Hitachi and multiple small OEMs.
In addition to cardiac trigger monitors, Ivy produces multiparameter patient monitors, and modular solutions for the healthcare market.
For more information: www.ivybiomedical


 February 13, 2026
February 13, 2026 









