February 1, 2013 — SoftVue, the whole breast ultrasound device created by two scientists from the Barbara Ann Karmanos Cancer Institute and Wayne State University School of Medicine, is currently undergoing review by the U.S. Food and Drug Administration (FDA) for market clearance. It is anticipated the first application clearance could come in the spring, with several other FDA submissions to follow over the next couple of years. This imaging tool has the potential to aid in detecting breast cancer earlier, especially in women with dense breasts.
Peter Littrup, M.D., and Neb Duric, Ph.D., of the Karmanos Cancer Institute and Wayne State University School of Medicine, and their team have been working on the SoftVue technology for more than a decade. The Karmanos Cancer Institute spun off a company in 2009 called Delphinus Medical Technologies to help secure funding to bring the device closer to commercialization.
"We are pleased to see the progress of SoftVue over the past 12 years, first with the concept followed by the prototype created at Karmanos a few years later," said Gerold Bepler, M.D., Ph.D., president and CEO of the Barbara Ann Karmanos Cancer Institute. "After years of dedication and hard work by co-creators Peter Littrup, M.D., and Neb Duric, Ph.D., along with their team, we are delighted that Karmanos has the first commercial grade SoftVue system to offer to our patients. Information obtained from this new SoftVue system will continue the next round of clinical research for use in diagnostic breast imaging.”
More than $32 million in funding, including venture capital and other grants, has helped turn SoftVue's technology into a commercial product – pending FDA clearance to market. The first round of venture capital funding included Michigan companies Arboretum Ventures, Altarum Institute, Beringea, LLC's InvestMichigan! Growth Capital Fund and North Coast Technology Investors. Other funding was received from several private donors along with grants from the National Institutes of Health, the Susan G. Komen Breast Cancer Foundation and the Michigan Economic Development Corp. The Karmanos Cancer Institute currently has the only commercial grade SoftVue system in the world.
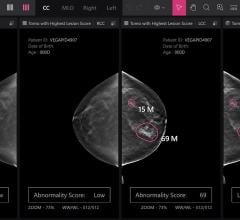
SoftVue uses ultrasound waves, which have the potential to aid in detecting early stages of breast cancer, even in women with dense breast tissue often not picked up by mammography. The interaction of sound waves with cancerous tissue yields a unique signature that can be measured using the SoftVue technology. The SoftVue system collects information not often detected by conventional ultrasound imaging, resulting in a more accurate and complete image of the tissue’s characteristics.
The SoftVue exam takes about one minute and produces images in less than 15 minutes. It does not involve the radiation or compression used in mammography and is a fraction of the cost of breast MRI (magnetic resonance imaging). With SoftVue, the breast is submerged in warm water and an ultrasound transducer ring surrounds the breast without touching it. The SoftVue system transmits and receives ultrasound signals around the entire breast, allowing it to capture detailed 3-D images. The system is able to perform repeated imaging, a necessary tool for biopsy, monitoring and treatment assessment.
Once market clearance is received for the first FDA submission, SoftVue systems will be produced for other medical centers that will take part in clinical studies needed to secure further FDA approvals. Up to now, SoftVue studies have been only for diagnostic purposes, showing the quality and safety of the technology compared to other breast ultrasound devices.
The company plans to carry out clinical trials involving approximately 15,000 to 20,000 subjects to support an FDA pre-market approval application, which, once approved, will give Delphinus the green light to sell SoftVue for breast cancer screening in the United States. Delphinus CEO William Greenway anticipates it could take until 2015, depending on the number of people participating in the clinical studies at various medical centers, as well as on the funding to manufacture the systems.
"Once we secure this first FDA clearance, we'll receive signed commitments from other health centers that have expressed interest in SoftVue," said Greenway. "The approximate cost for the SoftVue machine is $400,000, comparable to mammography. We anticipate that we'll need another $15 to $17 million in venture capital to carry out the trials and produce the machines, which will be manufactured in Michigan."
For more information: www.karmanos.org


 February 17, 2026
February 17, 2026